Spatial transcriptomics in bone mechanomics: Exploring the mechanoregulation of fracture healing in the era of spatial omics
- PMID: 39742473
- PMCID: PMC11694773
- DOI: 10.1126/sciadv.adp8496
Spatial transcriptomics in bone mechanomics: Exploring the mechanoregulation of fracture healing in the era of spatial omics
Abstract
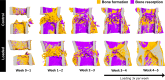
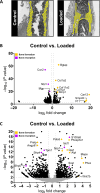
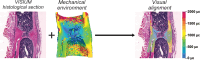
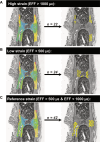
In recent decades, the field of bone mechanobiology has sought experimental techniques to unravel the molecular mechanisms governing the phenomenon of mechanically regulated fracture healing. Each cell within a fracture site resides within different local microenvironments characterized by different levels of mechanical strain; thus, preserving the spatial location of each cell is critical in relating cellular responses to mechanical stimuli. Our spatial transcriptomics-based "mechanomics" platform facilitates spatially resolved analysis of the molecular profiles of cells with respect to their local in vivo mechanical environment by integrating time-lapsed in vivo micro-computed tomography, spatial transcriptomics, and micro-finite element analysis. We investigate the transcriptomic responses of cells as a function of the local strain magnitude by identifying the differential expression of genes in regions of high and low strain within a fracture site. Our platform thus has the potential to address fundamental open questions within the field and to discover mechano-responsive targets to enhance fracture healing.
Figures




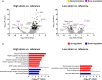
References
-
- J. Wolff, Das Gesetz der Transformation der Knochen (Verlag von August Hirschwald, 1892). [The law of bone remodeling].
-
- L. E. Claes, Mechanobiology of Fracture Healing: From Basic Science to Clinical Application, Springer Briefs in Bioengineering (Springer International Publishing, 2022); 10.1007/978-3-030-94082-9. - DOI
-
- S. W. Verbruggen, L. M. McNamara, Bone mechanobiology in health and disease, in Mechanobiology in Health and Disease (Elsevier, 2018), pp. 157–214; https://linkinghub.elsevier.com/retrieve/pii/B9780128129524000064.
-
- A. B. Castillo, J. C. Chen, C. R. Jacobs, Cellular and molecular mechanotransduction in bone, in Marcus and Feldman’s Osteoporosis (Elsevier, 2021), pp. 309–335; https://linkinghub.elsevier.com/retrieve/pii/B9780128130735000149.
MeSH terms
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

