Blood-derived APLP1+ extracellular vesicles are potential biomarkers for the early diagnosis of brain diseases
- PMID: 39742488
- PMCID: PMC11691634
- DOI: 10.1126/sciadv.ado6894
Blood-derived APLP1+ extracellular vesicles are potential biomarkers for the early diagnosis of brain diseases
Abstract
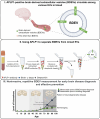
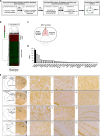
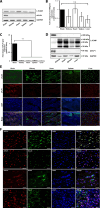
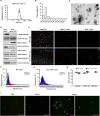
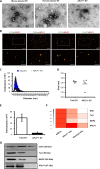
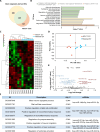
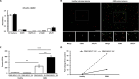
The early detection of neurodegenerative diseases necessitates the identification of specific brain-derived biomolecules in peripheral blood. In this context, our investigation delineates the role of amyloid precursor-like protein 1 (APLP1)-a protein predominantly localized in oligodendrocytes and neurons-as a previously unidentified biomarker in extracellular vesicles (EVs). Through rigorous analysis, APLP1+ EVs from human sera were unequivocally determined to be of cerebral origin. This assertion was corroborated by distinctive small RNA expression patterns of APLP1+ EVs. The miRNAs' putative targets within these EVs manifested pronounced expression in the brain, fortifying their neurospecific provenance. We subjected our findings to stringent validation using Thy-1 GFP M line mice, transgenic models wherein GFP expression is confined to hippocampal neurons. An amalgamation of these results with an exhaustive data analysis accentuates the potential of APLP1+ EVs as cerebrally originated biomarkers. Synthesizing our findings, APLP1+ EVs are postulated not merely as diagnostic markers but as seminal entities shaping the future trajectory of neurodegenerative disease diagnostics.
Figures
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

