Transcription factor clusters as information transfer agents
- PMID: 39742495
- PMCID: PMC11691696
- DOI: 10.1126/sciadv.adp3251
Transcription factor clusters as information transfer agents
Abstract
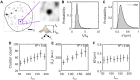
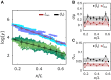
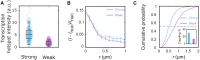
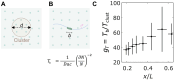
Deciphering how genes interpret information from transcription factor (TF) concentrations within the cell nucleus remains a fundamental question in gene regulation. Recent advancements have revealed the heterogeneous distribution of TF molecules, posing challenges to precisely decoding concentration signals. Using high-resolution single-cell imaging of the fluorescently tagged TF Bicoid in living Drosophila embryos, we show that Bicoid accumulation in submicrometer clusters preserves the spatial information of the maternal Bicoid gradient. These clusters provide precise spatial cues through intensity, size, and frequency. We further discover that Bicoid target genes colocalize with these clusters in an enhancer-binding affinity-dependent manner. Our modeling suggests that clustering offers a faster sensing mechanism for global nuclear concentrations than freely diffusing TF molecules detected by simple enhancers.
Figures


Update of
-
Transcription factor clusters as information transfer agents.ArXiv [Preprint]. 2024 Nov 6:arXiv:2403.02943v3. ArXiv. 2024. Update in: Sci Adv. 2025 Jan 3;11(1):eadp3251. doi: 10.1126/sciadv.adp3251. PMID: 38495568 Free PMC article. Updated. Preprint.
Similar articles
-
Transcription factor clusters as information transfer agents.ArXiv [Preprint]. 2024 Nov 6:arXiv:2403.02943v3. ArXiv. 2024. Update in: Sci Adv. 2025 Jan 3;11(1):eadp3251. doi: 10.1126/sciadv.adp3251. PMID: 38495568 Free PMC article. Updated. Preprint.
-
Impacts of the ubiquitous factor Zelda on Bicoid-dependent DNA binding and transcription in Drosophila.Genes Dev. 2014 Mar 15;28(6):608-21. doi: 10.1101/gad.234534.113. Genes Dev. 2014. PMID: 24637116 Free PMC article.
-
Synthetic enhancer design by in silico compensatory evolution reveals flexibility and constraint in cis-regulation.BMC Syst Biol. 2017 Nov 29;11(1):116. doi: 10.1186/s12918-017-0485-2. BMC Syst Biol. 2017. PMID: 29187214 Free PMC article.
-
Constraints and limitations on the transcriptional response downstream of the Bicoid morphogen gradient.Curr Top Dev Biol. 2020;137:119-142. doi: 10.1016/bs.ctdb.2019.12.002. Epub 2020 Jan 17. Curr Top Dev Biol. 2020. PMID: 32143741 Review.
-
The mother-to-child transition.Dev Cell. 2007 Jun;12(6):847-9. doi: 10.1016/j.devcel.2007.05.009. Dev Cell. 2007. PMID: 17543857 Review.
Cited by
-
Bicoid-nucleosome competition sets a concentration threshold for transcription constrained by genome replication.bioRxiv [Preprint]. 2024 Dec 12:2024.12.10.627802. doi: 10.1101/2024.12.10.627802. bioRxiv. 2024. Update in: Cell Rep. 2025 Aug 26;44(8):116121. doi: 10.1016/j.celrep.2025.116121. PMID: 39713295 Free PMC article. Updated. Preprint.
-
Both the transcriptional activator, Bcd, and repressor, Cic, form small mobile oligomeric clusters.Biophys J. 2025 Mar 18;124(6):980-995. doi: 10.1016/j.bpj.2024.08.011. Epub 2024 Aug 20. Biophys J. 2025. PMID: 39164967
-
Enhancer regulation in cancer: from epigenetics to m6A RNA modification.Arch Pharm Res. 2025 Aug;48(7-8):706-735. doi: 10.1007/s12272-025-01561-1. Epub 2025 Aug 19. Arch Pharm Res. 2025. PMID: 40830299 Review.
-
Biological physics to uncover cell signaling.Biophys Rev. 2025 Apr 10;17(2):271-283. doi: 10.1007/s12551-025-01308-8. eCollection 2025 Apr. Biophys Rev. 2025. PMID: 40376425 Review.
References
-
- Spitz F., Furlong E. E., Transcription factors: From enhancer binding to developmental control. Nat. Rev. Genet. 13, 613–626 (2012). - PubMed
-
- Trojanowski J., Rippe K., Transcription factor binding and activity on chromatin. Curr. Opin. Systs. Biol. 31, 100438 (2022).
-
- Rosenfeld N., Young J. W., Alon U., Swain P. S., Elowitz M. B., Gene regulation at the single-cell level. Science 307, 1962–1965 (2005). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

