CD154 blockade effectively controls antibody-mediated rejection in highly sensitized nonhuman primate kidney transplant recipients
- PMID: 39742504
- PMCID: PMC11797747
- DOI: 10.1126/scitranslmed.adn8130
CD154 blockade effectively controls antibody-mediated rejection in highly sensitized nonhuman primate kidney transplant recipients
Abstract
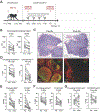
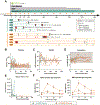
Current desensitization and maintenance immunosuppression regimens for kidney transplantation in sensitized individuals show limited ability to control the posttransplant humoral response, resulting in high rates of antibody-mediated rejection (ABMR) and graft failure. Here, we showed that anti-CD154 monoclonal antibody (mAb)-based immunosuppression more effectively controlled allograft rejection and humoral rebound in a highly sensitized nonhuman primate kidney transplantation model compared with tacrolimus-based standard-of-care (SOC) immunosuppression. Desensitization with an anti-CD154 mAb (5C8) and a proteasome inhibitor led to decreased donor-specific antibodies (DSAs) and disruption of lymph node germinal centers with reduction of proliferating, memory, and class-switched B cells as well as T follicular helper cells. After transplant, the nonhuman primates maintained on 5C8-based immunosuppression had significantly better survival compared with those maintained on SOC immunosuppression (135.2 days versus 32.8 days, P = 0.013). The 5C8-treated group demonstrated better suppression of DSAs after transplant, more robust suppression of B cell populations, and better induction of regulatory T cells. Fewer infectious and welfare complications, including viral reactivation and weight loss, were also observed with 5C8-based immunosuppression compared with SOC immunosuppression. Therefore, anti-CD154 mAbs may improve kidney transplant outcomes through better control of posttransplant immune responses. The superior efficacy of anti-CD154 mAb-based immunosuppression over tacrolimus-based SOC seen in this highly sensitized NHP transplant model suggests that anti-CD154 mAbs could potentially be used to desensitize and treat highly sensitized patients receiving kidney transplantation.
Conflict of interest statement
Competing interests
The authors declare that they have no competing interests.
Figures
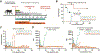

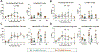

References
-
- Muduma G, Odeyemi I, Smith-Palmer J, Pollock RF, Review of the Clinical and Economic Burden of Antibody-Mediated Rejection in Renal Transplant Recipients. Adv Ther 33, 345–356 (2016). - PubMed
-
- Port FK, Wolfe RA, Mauger EA, Berling DP, Jiang K, Comparison of survival probabilities for dialysis patients vs cadaveric renal transplant recipients. JAMA 270, 1339–1343 (1993). - PubMed
-
- Stewart DE, Wilk AR, Toll AE, Harper AM, Lehman RR, Robinson AM, Noreen SA, Edwards EB, Klassen DK, Measuring and monitoring equity in access to deceased donor kidney transplantation. Am J Transplant 18, 1924–1935 (2018). - PubMed
-
- Lentine KL, Smith JM, Hart A, Miller J, Skeans MA, Larkin L, Robinson A, Gauntt K, Israni AK, Hirose R, Snyder JJ, OPTN/SRTR 2020 Annual Data Report: Kidney. Am J Transplant 22 Suppl 2, 21–136 (2022). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

