G6PC2 controls glucagon secretion by defining the set point for glucose in pancreatic α cells
- PMID: 39742505
- PMCID: PMC12212188
- DOI: 10.1126/scitranslmed.adi6148
G6PC2 controls glucagon secretion by defining the set point for glucose in pancreatic α cells
Abstract
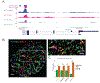
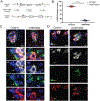
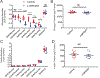
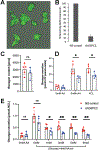
Elevated glucagon concentrations have been reported in patients with type 2 diabetes (T2D). A critical role for α cell-intrinsic mechanisms in regulating glucagon secretion was previously established through genetic manipulation of the glycolytic enzyme glucokinase (GCK) in mice. Genetic variation at the glucose-6-phosphatase catalytic subunit 2 (G6PC2) locus, encoding an enzyme that opposes GCK, has been reproducibly associated with fasting blood glucose and hemoglobin A1c. Here, we found that trait-associated variants in the G6PC2 promoter are located in open chromatin not just in β but also in α cells and documented allele-specific G6PC2 expression of linked variants in human α cells. Using α cell-specific gene ablation of G6pc2 in mice, we showed that this gene plays a critical role in controlling glucose suppression of amino acid-stimulated glucagon secretion independent of alterations in insulin output, islet hormone content, or islet morphology, findings that we confirmed in primary human α cells. Collectively, our data demonstrate that G6PC2 affects glycemic control via its action in α cells and possibly suggest that G6PC2 inhibitors might help control blood glucose through a bihormonal mechanism.
Conflict of interest statement
Competing interests: The authors declare that they have no competing interests, no consulting agreements and that no patent has been filed regarding the data presented in this study.
Figures



References
-
- Gerich JE, Charles MA, Grodsky GM, Regulation of pancreatic insulin and glucagon secretion. Annu Rev Physiol 38, 353–388 (1976). - PubMed
-
- Quesada I, Tuduri E, Ripoll C, Nadal A, Physiology of the pancreatic alpha-cell and glucagon secretion: role in glucose homeostasis and diabetes. J Endocrinol 199, 5–19 (2008). - PubMed
-
- Zhang P, Zhang X, Brown J, Vistisen D, Sicree R, Shaw J, Nichols G, Global healthcare expenditure on diabetes for 2010 and 2030. Diabetes Res Clin Pract 87, 293–301 (2010). - PubMed
-
- Unger RH, Orci L, The essential role of glucagon in the pathogenesis of diabetes mellitus. Lancet 1, 14–16 (1975). - PubMed
-
- D'Alessio D, The role of dysregulated glucagon secretion in type 2 diabetes. Diabetes Obes Metab 13 Suppl 1, 126–132 (2011). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

