Genetic variants of accessory proteins and G proteins in human genetic disease
- PMID: 39743506
- PMCID: PMC11854058
- DOI: 10.1080/10408363.2024.2431853
Genetic variants of accessory proteins and G proteins in human genetic disease
Abstract
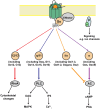
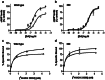
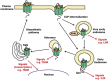
We present a series of three articles on the genetics and pharmacogenetics of G protein- coupled receptors (GPCR). In the first article, we discuss genetic variants of the G protein subunits and accessory proteins that are associated with human phenotypes; in the second article, we build upon this to discuss "G protein-coupled receptor (GPCR) gene variants and human genetic disease" and in the third article, we survey "G protein-coupled receptor pharmacogenomics". In the present article, we review the processes of ligand binding, GPCR activation, inactivation, and receptor trafficking to the membrane in the context of human genetic disease resulting from pathogenic variants of accessory proteins and G proteins. Pathogenic variants of the genes encoding G protein α and β subunits are examined in diverse phenotypes. Variants in the genes encoding accessory proteins that modify or organize G protein coupling have been associated with disease; these include the contribution of variants of the regulator of G protein signaling (RGS) to hypertension; the role of variants of activator of G protein signaling type III in phenotypes such as hypoxia; the contribution of variation at the RGS10 gene to short stature and immunological compromise; and the involvement of variants of G protein-coupled receptor kinases (GRKs), such as GRK4, in hypertension. Variation in genes that encode proteins involved in GPCR signaling are outlined in the context of the changes in structure and function that may be associated with human phenotypes.
Keywords: G protein; G protein-coupled receptor (GPCR); accessory protein; genetics; pharmacogenetics.
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures




References
-
- Liu X, Davis D, Segaloff DL.. Disruption of potential sites for N-linked glycosylation does not impair hormone binding to the lutropin/choriogonadotropin receptor if Asn-173 is left intact. J Biol Chem. 1993;268(3):1513–1516. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
