Centrophilic retrotransposon integration via CENH3 chromatin in Arabidopsis
- PMID: 39743586
- PMCID: PMC11735389
- DOI: 10.1038/s41586-024-08319-7
Centrophilic retrotransposon integration via CENH3 chromatin in Arabidopsis
Abstract
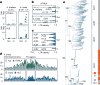
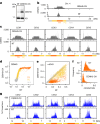
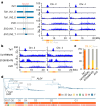
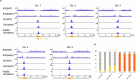
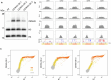
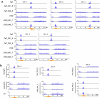
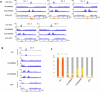
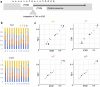
In organisms ranging from vertebrates to plants, major components of centromeres are rapidly evolving repeat sequences, such as tandem repeats (TRs) and transposable elements (TEs), which harbour centromere-specific histone H3 (CENH3)1,2. Complete centromere structures recently determined in human and Arabidopsis suggest frequent integration and purging of retrotransposons within the TR regions of centromeres3-5. Despite the high impact of 'centrophilic' retrotransposons on the paradox of rapid centromere evolution, the mechanisms involved in centromere targeting remain poorly understood in any organism. Here we show that both Ty3 and Ty1 long terminal repeat retrotransposons rapidly turnover within the centromeric TRs of Arabidopsis species. We demonstrate that the Ty1/Copia element Tal1 (Transposon of Arabidopsis lyrata 1) integrates de novo into regions occupied by CENH3 in Arabidopsis thaliana, and that ectopic expansion of the CENH3 region results in spread of Tal1 integration regions. The integration spectra of chimeric TEs reveal the key structural variations responsible for contrasting chromatin-targeting specificities to centromeres versus gene-rich regions, which have recurrently converted during the evolution of these TEs. Our findings show the impact of centromeric chromatin on TE-mediated rapid centromere evolution, with relevance across eukaryotic genomes.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures





References
-
- Henikoff, S., Ahmad, K. & Malik, H. S. The centromere paradox: stable inheritance with rapidly evolving DNA. Science293, 1098–1102 (2001). - PubMed
-
- Malik, H. S. & Henikoff, S. Major evolutionary transitions in centromere complexity. Cell138, 1067–1082 (2009). - PubMed
-
- Wlodzimierz, P. et al. Cycles of satellite and transposon evolution in Arabidopsis centromeres. Nature618, 557–565 (2023). - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

