Case report: Improving quality of life through hyaluronic acid-based fillers after orbital cancer treatment
- PMID: 39744006
- PMCID: PMC11688356
- DOI: 10.3389/fonc.2024.1501556
Case report: Improving quality of life through hyaluronic acid-based fillers after orbital cancer treatment
Abstract
Background: Myoepithelial carcinoma is a very rare yet aggressive tumor in children. Surgical intervention and local radiotherapy often lead to post-therapy complications, affecting both the aesthetic and functional quality of life in survivors. Hyaluronic acid (HA) dermal fillers offer a minimally invasive option to improve the appearance and quality of life for these patients once they are declared tumor-free.
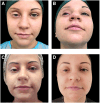
Case presentation: We present the case of an 18-year-old girl with a history of myoepithelial carcinoma in the right upper orbit, diagnosed at the age of 8. The patient underwent surgery to remove the tumor and lacrimal gland, followed by chemotherapy and radiotherapy. A complete response to treatment was achieved, and the patient was monitored with regular clinical and radiological exams for 5 years, after which she was declared tumor-free and followed for late effects of therapy. Post-surgical radiotherapy resulted in atrophy of the upper orbital frame and functional complications. The patient exhibited upper eyelid retraction, ptosis, continuous lacrimation, and conjunctival redness. Ten years after treatment, the patient underwent dermal filler injections using Aliaxin® Essential Volume (AEV) and Aliaxin® Superior Volume (ASV) to address the aesthetic impairment of the upper right orbit. ASV was administered using a 22G x 50mm cannula on the periosteum of the superior orbital frame, entering from the outer canthus. AEV was injected with a cannula into the muscle, also entering from the outer canthus. Before treatment, the patient exhibited upper eyelid retraction, ptosis, continuous lacrimation, and conjunctival redness. Following the injections, improvements were observed in all pre-treatment symptoms. The closing ability of the upper eyelid was restored, along with superior orbital volume and symmetry. Enhanced eyelid function improved eye hydration, reduced redness in the conjunctiva, and led to better vision and overall quality of life.
Conclusion: To our knowledge, this is the first reported case of using dermal fillers to treat ocular changes resulting from cancer treatment. Injections of AEV and ASV provided both aesthetic and functional improvements.
Keywords: aesthetic medicine; dermal filler; hyaluronic acid; myoepithelial carcinoma; post-surgical complication.
Copyright © 2024 Egidi, Valentini and Schiavetti.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- American Cancer Society . Cancer Treatment & Survivorship Facts & Figures 2022-2024. Atlanta: American Cancer Society; (2022).
-
- Board PPTE . Late effects of treatment for childhood cancer (PDQ®). In: PDQ Cancer Information Summaries. National Cancer Institute (US; (2004).
Publication types
LinkOut - more resources
Full Text Sources

