Differentiation ability of hematopoietic stem cells and mesenchymal stem cells isolated from human peripheral blood
- PMID: 39744010
- PMCID: PMC11688275
- DOI: 10.3389/fcell.2024.1450543
Differentiation ability of hematopoietic stem cells and mesenchymal stem cells isolated from human peripheral blood
Abstract
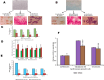
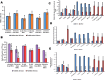
Human hematopoietic stem cells (HSCs) and mesenchymal stem cells (MSCs) are the major stem cells of the bone marrow and are usually isolated from the peripheral blood. In the present study, we isolated these stem cells by an apheresis method from a donor who was administered granulocyte colony-stimulating factor (G-CSF). In vitro propagation of these stem cells showed a plastic-adherence property expressing CD73 and CD105 surface markers, which is a characteristic feature of MSCs. HSCs are non-adherent cells growing as a suspension culture, expressing CD150, CD133, CD34, and CD45 on their surface, which regulate the quiescence nature, and they derive energy from anaerobic glycolysis. The HSCs grow slowly compared to MSCs, are more viable, and survive for long periods under in vitro conditions, which are due to the expression of telomerase, BCL2, and Notch1 genes. The poor viability of MSCs in the culture due to the prominent expression of apoptotic genes BAX, caspase-3, and caspase-9 leads to rapid apoptosis. This was evident even in cells (astrocytes, osteocytes, and beta cells of the islets of Langerhans) differentiated from HSCs and MSCs, thus highlighting the importance of HSCs, the naive stem cells, in regeneration of tissues.
Keywords: differentiation ability; human hematopoietic stem cells; mesenchymal stem cells; peripheral blood stem cells; regenerative medicine.
Copyright © 2024 Samundeshwari, Kattaru, Kodavala, Chandrasekhar and Sarma.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

