Development and assessment of a novel magnetic nanoparticle antibody-conjugate and aptamer-based assay (MNp-Ab-Ap assay) for the rapid diagnosis of pleural tuberculosis
- PMID: 39744100
- PMCID: PMC11667566
- DOI: 10.7150/ntno.95332
Development and assessment of a novel magnetic nanoparticle antibody-conjugate and aptamer-based assay (MNp-Ab-Ap assay) for the rapid diagnosis of pleural tuberculosis
Abstract
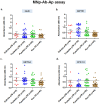
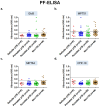
Background: Pleural tuberculosis (pTB) is a diagnostic challenge because of its non-specific clinical features, lack of accurate diagnostic tools and paucibacillary nature of the disease. Methods: We, here describe the development of a novel magnetic nanoparticle antibody-conjugate and aptamer-based assay (MNp-Ab-Ap assay) targeting 4 different Mycobacterium tuberculosis (M. tb.) antigens (GlcB, MPT51, MPT64 and CFP-10) for pTB diagnosis. The MNp-Ab-Ap assay was developed by conjugating polyclonal antibodies on the surface of magnetic nanoparticles (MNPs) by using EDC-NHS chemistry. These conjugated MNPs were used to capture M. tb. antigens present in the pleural fluid samples. The resulting antigen-antibody complex was detected by antigen-specific 5'-biotinylated aptamers. All assays were standardized using samples of the 'Development set' (n=17) and evaluated in the 'Validation set' (n=114) in a blinded manner. Patient categorization was done using a 'Composite Reference Standard'. Assay cut-offs were determined from the 'Development set' (n=17; 'Definite & Probable' pTB; n=9 and 'Non-TB'; n=8) by calculating mean+3SD of OD450 values of the 'Non-TB' group and applied to 'Validation set' (n=114; 'Definite' pTB; n=8, 'Probable' pTB; n=34, 'Possible' pTB; n=28 and 'Non-TB'; n=44). Results: Out of the 4 assays, MPT51-based MNp-Ab-Ap assay performed the best with 66.6% (95%CI;50.4-80.4) sensitivity and 95.4% (95%CI;85.1-99.4) specificity in the combined 'Definite and Probable' pTB group. Xpert MTB/RIF assay detected only six samples in the 'Validation set'. Binary logistic regression analysis indicated that MPT51-based MNp-Ab-Ap assay provided an incremental advantage over the existing diagnostic algorithm for pTB. Conclusions: We conclude that MPT51-based MNp-Ab-Ap assay is a novel technique that can pave the way towards rapid and accurate diagnosis of pTB.
© The author(s).
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures



Similar articles
-
Evaluation of Mycobacterium tuberculosis derived cell-free DNA using pleural fluid and paired plasma samples for the diagnosis of pleural tuberculosis.Tuberculosis (Edinb). 2023 Sep;142:102369. doi: 10.1016/j.tube.2023.102369. Epub 2023 Jul 8. Tuberculosis (Edinb). 2023. PMID: 37536090
-
Utility of pleural fluid-derived extracellular vesicles as a source of Mycobacterium tuberculosis antigens MPT51 and MPT64 for pleural TB diagnosis: a proof-of-concept study.Tuberculosis (Edinb). 2025 Jan;150:102578. doi: 10.1016/j.tube.2024.102578. Epub 2024 Nov 19. Tuberculosis (Edinb). 2025. PMID: 39647431
-
A novel aptamer-based test for the rapid and accurate diagnosis of pleural tuberculosis.Anal Biochem. 2019 Jan 1;564-565:80-87. doi: 10.1016/j.ab.2018.10.019. Epub 2018 Oct 22. Anal Biochem. 2019. PMID: 30352198
-
Challenges in pleural tuberculosis diagnosis: existing reference standards and nucleic acid tests.Future Microbiol. 2017 Oct;12:1201-1218. doi: 10.2217/fmb-2017-0028. Epub 2017 Sep 15. Future Microbiol. 2017. PMID: 28972418 Review.
-
Xpert MTB/RIF Ultra and Xpert MTB/RIF assays for extrapulmonary tuberculosis and rifampicin resistance in adults.Cochrane Database Syst Rev. 2021 Jan 15;1(1):CD012768. doi: 10.1002/14651858.CD012768.pub3. Cochrane Database Syst Rev. 2021. PMID: 33448348 Free PMC article.
References
-
- Maurya AK, Kant S, Kushwaha RA, Nag VL, Kumar M, Dhole TN. The advantage of using IS6110-PCR vs. BACTEC culture for rapid detection of Mycobacterium tuberculosis from pleural fluid in northern India. Bioscience trends. 2011;5(4):159–64. - PubMed
-
- Tyagi S, Sharma N, Tyagi JS, Haldar S. Challenges in pleural tuberculosis diagnosis: existing reference standards and nucleic acid tests. Future microbiology. 2017;12:1201–18. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

