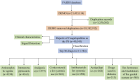
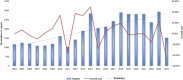
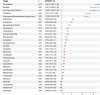
Drug-induced coagulopathies: a real-world pharmacovigilance study using the FDA adverse event reporting system
- PMID: 39744131
- PMCID: PMC11688381
- DOI: 10.3389/fphar.2024.1486422
Drug-induced coagulopathies: a real-world pharmacovigilance study using the FDA adverse event reporting system
Abstract
Background: This study aims to investigate adverse drug reaction signals associated with coagulopathies through data mining using the Adverse Event Reporting System (FAERS) of the US Food and Drug Administration. Prompt identification of high-risk drugs provides a valuable basis for enhancing clinical drug safety.
Methods: The adverse event reports related to coagulopathies from Q1 2004 to Q2 2024 were extracted from the ASCII data packages in FAERS. The reporting odds ratio (ROR), proportional reporting ratio (PRR), and Bayesian confidence propagation neural network (BCPNN) were used to identify adverse drug reaction signals associated with coagulopathies.
Results: During the reporting period, 40,545 reports were retrieved, with a slightly higher proportion of females than males. Among the top 30 drugs associated with the occurrence of coagulopathies, 24 drugs exhibited positive signals in risk analysis. Based on the individual drug reporting odds ratio (95% confidence interval) as a measure of risk signal strength, the top five drugs are as follows: gemcitabine [ROR (95% CI):16.87 (15.83-17.98)], busulfan [ROR (95% CI):15.51 (13.69-17.58)], anti-thymocyte globulin [ROR (95% CI):15.49 (13.49-17.78)], tacrolimus [ROR (95% CI):12.7 (11.57-13.95)], etonogestrel and ethinylestradiol vaginal ring [ROR (95% CI):11.88 (10.95-12.89)]. After categorizing the drugs, the strongest risk signal is sex hormones and modulators of the genital system [ROR (95% CI):11.88 (10.95-12.89)], followed by analgesics [ROR (95%CI): 6.73 (6.38-7.1)], immunosuppressants [ROR (95% CI):3.91 (3.76-4.05)], antineoplastic agents [ROR (95% CI):3.33 (3.22-3.45)], corticosteroids for systemic use [ROR (95% CI): 2.94 (2.73-3.18)], antiepileptics [ROR (95% CI):1.93 (1.71-2.18)], drugs used in diabetes [ROR (95% CI):1.5 (1.34-1.67)], antibacterials for systemic use [ROR (95% CI):1.46 (1.28-1.68)].
Conclusion: Our findings indicate that multiple drugs are associated with an increased risk of coagulopathies. From the pharmacovigilance perspective, proactive analysis of these drugs aids in clinical monitoring and enhances risk identification of coagulopathies.
Keywords: FAERS database; adverse event; coagulopathies; data mining; disproportionality analyses; pharmacovigilance.
Copyright © 2024 Lu, Xu and Zhu.
Conflict of interest statement
The authors declare that the research was conducted without any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
LinkOut - more resources
Full Text Sources