Liver B Cells Promotes MASLD Progression via the Apelin/APLNR System
- PMID: 39744169
- PMCID: PMC11659828
- DOI: 10.7150/ijms.101492
Liver B Cells Promotes MASLD Progression via the Apelin/APLNR System
Abstract
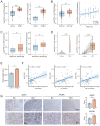
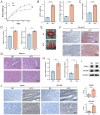
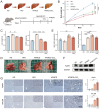
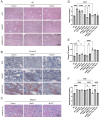
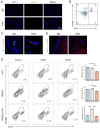
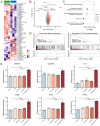
Aims: Investigate the role of the apelin/APLNR axis in metabolic dysfunction-associated steatotic liver disease (MASLD), focusing on the progression from metabolic dysfunction-associated simple steatotic liver (MASS) to metabolic dysfunction-associated steatohepatitis (MASH) and fibrosis, with emphasis on liver B cells. Methods: Serum samples from MASLD patients and liver tissues from hepatocellular carcinoma patients were collected to measure apelin and APLNR protein expression. C57BL/6J mouse models of varying MASLD stages were developed using a high-fat diet and CCl4. RNA sequencing was used to study the apelin/APLNR axis's regulatory functions in the Raji B cell line. Results: Bioinformatic and clinical analyses show that apelin and APLNR are up-regulated in MASLD, correlating with disease severity. Animal models demonstrate that apelin and ML221 injections affect liver steatosis, inflammation, and fibrosis. Sequencing and RT-PCR in Raji cells indicate that the apelin/APLNR axis promotes the expression of inflammatory cytokines and extracellular matrix molecules. Conclusion: The apelin/APLNR axis is crucial in MASLD progression. Targeting this axis may offer therapeutic potential to modulate B cell function and mitigate MASLD advancement.
Keywords: B cells; apelin/APLNR; metabolic dysfunction-associated steatotic liver disease.
© The author(s).
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures
Similar articles
-
RASSF4 Attenuates Metabolic Dysfunction-Associated Steatotic Liver Disease Progression via Hippo Signaling and Suppresses Hepatocarcinogenesis.Cell Mol Gastroenterol Hepatol. 2024;18(2):101348. doi: 10.1016/j.jcmgh.2024.04.005. Epub 2024 Apr 30. Cell Mol Gastroenterol Hepatol. 2024. PMID: 38697356 Free PMC article.
-
Protective Effects of Hepatocyte Stress Defenders, Nrf1 and Nrf2, against MASLD Progression.Int J Mol Sci. 2024 Jul 24;25(15):8046. doi: 10.3390/ijms25158046. Int J Mol Sci. 2024. PMID: 39125617 Free PMC article.
-
Apelin and the gut microbiome: Potential interaction in human MASLD.Dig Liver Dis. 2024 Jun;56(6):932-940. doi: 10.1016/j.dld.2023.11.023. Epub 2023 Dec 11. Dig Liver Dis. 2024. PMID: 38087672
-
The microenvironment in the development of MASLD-MASH-HCC and associated therapeutic in MASH-HCC.Front Immunol. 2025 Apr 30;16:1569915. doi: 10.3389/fimmu.2025.1569915. eCollection 2025. Front Immunol. 2025. PMID: 40370443 Free PMC article. Review.
-
Role of the type 3 cytokines IL-17 and IL-22 in modulating metabolic dysfunction-associated steatotic liver disease.Front Immunol. 2024 Aug 2;15:1437046. doi: 10.3389/fimmu.2024.1437046. eCollection 2024. Front Immunol. 2024. PMID: 39156888 Free PMC article. Review.
Cited by
-
Dynamic crosstalk between HSCs and liver microenvironment: multicellular interactions in the regulation of liver fibrosis.Front Cell Dev Biol. 2025 Jul 21;13:1635763. doi: 10.3389/fcell.2025.1635763. eCollection 2025. Front Cell Dev Biol. 2025. PMID: 40761743 Free PMC article. Review.
References
-
- Cai J, Xu M, Zhang X. et al. Innate Immune Signaling in Nonalcoholic Fatty Liver Disease and Cardiovascular Diseases. Annu Rev Pathol. 2019;14:153–84. - PubMed
-
- Ahmed A, Wong RJ, Harrison SA. Nonalcoholic Fatty Liver Disease Review: Diagnosis, Treatment, and Outcomes. Clin Gastroenterol Hepatol. 2015;13:2062–70. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

