A comparative analysis of Marburg virus-infected bat and human models from public high-throughput sequencing data
- PMID: 39744175
- PMCID: PMC11659840
- DOI: 10.7150/ijms.100696
A comparative analysis of Marburg virus-infected bat and human models from public high-throughput sequencing data
Abstract
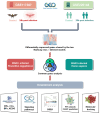
Marburg virus (MARV) disease (MVD) is an uncommon yet serious viral hemorrhagic fever that impacts humans and non-human primates. In humans, infection by the MARV is marked by rapid onset, high transmissibility, and elevated mortality rates, presenting considerable obstacles to the development of vaccines and treatments. Bats, particularly Rousettus aegyptiacus, are suspected to be natural hosts of MARV. Previous research reported asymptomatic MARV infection in bats, in stark contrast to the severe responses observed in humans and other primates. Recent MARV outbreaks highlight significant public health concerns, underscoring the need for gene expression studies during MARV progression. To investigate this, we employed two models from the Gene Expression Omnibus, including kidney cells from Rousettus aegyptiacus and primary proximal tubular cells from Homo sapiens. These models were chosen to identify changes in gene expression profiles and to examine co-regulated genes and pathways involved in MARV disease progression. Our analysis of differentially expressed genes (DEGs) revealed that these genes are mainly associated with pathways related to the complement system, innate immune response via interferons (IFNs), Wnt/β-catenin signaling, and Hedgehog signaling, which played crucial roles in MARV infection across both models. Furthermore, we also identified several potential compounds that may be useful against MARV infection. These findings offer valuable insights into the mechanisms underlying MARV's pathophysiology and suggest potential strategies for preventing transmission, managing post-infection effects, and developing future vaccines.
Keywords: Homo sapiens; Marburg virus (MARV); Rousettus aegyptiacus; bioinformatics; zoonotic disease.
© The author(s).
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures



Similar articles
-
Oral shedding of Marburg virus in experimentally infected Egyptian fruit bats (Rousettus aegyptiacus).J Wildl Dis. 2015 Jan;51(1):113-24. doi: 10.7589/2014-08-198. J Wildl Dis. 2015. PMID: 25375951 Free PMC article.
-
Transcriptional signatures of Ebola and Marburg virus infection in a bat-immune-system (BIS) mouse model.Adv Virus Res. 2025;122:19-59. doi: 10.1016/bs.aivir.2025.03.006. Epub 2025 Apr 19. Adv Virus Res. 2025. PMID: 40701747
-
Characterization of Ravn virus viral shedding dynamics in experimentally infected Egyptian rousette bats (Rousettus aegypticus).J Virol. 2025 May 20;99(5):e0004525. doi: 10.1128/jvi.00045-25. Epub 2025 Apr 23. J Virol. 2025. PMID: 40265897 Free PMC article.
-
From protein to immunology: comprehensive insights into Marburg virus vaccines, mechanism, and application.Arch Microbiol. 2025 Mar 1;207(4):74. doi: 10.1007/s00203-025-04277-4. Arch Microbiol. 2025. PMID: 40025302 Review.
-
Pathogenicity and virulence of Marburg virus.Virulence. 2022 Dec;13(1):609-633. doi: 10.1080/21505594.2022.2054760. Virulence. 2022. PMID: 35363588 Free PMC article. Review.
Cited by
-
Integration of multi-omics and single-cell transcriptome reveals mitochondrial outer membrane protein-2 (MTX-2) as a prognostic biomarker and characterizes ubiquinone metabolism in lung adenocarcinoma.J Cancer. 2025 Apr 13;16(7):2401-2420. doi: 10.7150/jca.106902. eCollection 2025. J Cancer. 2025. PMID: 40302794 Free PMC article.
References
-
- Falzarano D, Feldmann H. Marburg Virus. In: Mahy BWJ, Van Regenmortel MHV, editors. Encyclopedia of Virology (Third Edition). Oxford: Academic Press. 2008. p. 272-80.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

