Hypoxic conditions affect transcriptome of endometrial stromal cells in endometriosis and promote TGFBI axis
- PMID: 39744178
- PMCID: PMC11688238
- DOI: 10.3389/fendo.2024.1465393
Hypoxic conditions affect transcriptome of endometrial stromal cells in endometriosis and promote TGFBI axis
Abstract
Background: Endometriosis is characterized by the ectopic growth of endometrial-like cells, causing chronic pelvic pain, adhesions and impaired fertility in women of reproductive age. Usually, these lesions grow in the peritoneal cavity in a hypoxic environment. Hypoxia is known to affect gene expression and protein kinase (PK) activity. We aimed to explore the changes in the transcriptome and PK activity characteristic of eutopic and ectopic endometrium in endometriosis under hypoxia.
Methods: Eutopic (EuESCs) and ectopic (EcESCs) endometrial stromal cells were exposed to hypoxia (1% O2) or normoxia (20% O2) for 48 hours. We assessed PK activity and examined transcriptome using mRNA-seq in cells cultured under hypoxic or normoxic conditions. Enzyme-linked immunosorbent assay, quantitative reverse transcription-PCR and immunohistochemistry were performed for the downstream analysis of Transforming Growth Factor Beta Induced (TGFBI) expression.
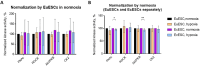
Results: The kinase assay revealed a minor decrease in cAMP-dependent PK (PKAc) and Akt activity and a trend towards an increase in Rho-dependent PK (ROCK) activity in response to exposure to hypoxic conditions in EcESCs. A wider examination of the hypoxia-mediated changes in transcriptomes of cultured cells revealed that the genes related to aerobic glycolysis and cellular metabolism were upregulated in EuESCs exposed to hypoxia. In contrast, EcESCs had a single differentially expressed gene (TGFBI) upregulated under hypoxic conditions. This gene was also found to be overexpressed in EuESCs exposed to hypoxia vs normoxia, and in EcESCs vs EuESCs in normoxia. The level of secreted TGFBI in the spent culture media was accordingly high in the EcESC cultures and in the EuESC culture exposed to hypoxia. In the eutopic endometrial tissue biopsies, TGFBI mRNA and protein expression depended on the menstrual cycle phase, with higher levels observed in the proliferative phase. TGFBI staining showed the protein localized to the stroma and around the blood vessels. In the secretory phase, TGFBI protein expression was stronger in ectopic endometrium compared to paired eutopic endometrium.
Conclusions: Within this study, we showed hypoxia-mediated transcriptome changes characteristic of EuESCs and EcESCs and identified TGFBI as a potential therapeutic target for endometriosis due to its role in fibrosis and angiogenesis.
Keywords: TGFBI; endometriosis; hypoxia; peritoneal lesions; stromal cell cultures; transcriptomics.
Copyright © 2024 Sarsenova, Boggavarapu, Kask, Modhukur, Samuel, Karro, Gemzell-Danielsson, Lalitkumar, Salumets, Peters and Lavogina.
Conflict of interest statement
Authors MS, KK, VM, KS, AS, MP, and DL were employed by the company Celvia CC AS. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

