Influence of nonalcoholic fatty liver disease on the therapeutic effect of nucleoside (acid) analogs for hepatitis B virus
- PMID: 39744194
- PMCID: PMC11686536
- DOI: 10.4254/wjh.v16.i12.1395
Influence of nonalcoholic fatty liver disease on the therapeutic effect of nucleoside (acid) analogs for hepatitis B virus
Abstract
Background: The effect of nonalcoholic fatty liver disease (NAFLD) on the efficacy of nucleoside analogues (NAs) in antiviral therapy for patients with chronic hepatitis B (CHB) remains controversial.
Aim: To investigate the influence of NAFLD on virological response in CHB patients undergoing NAs treatment.
Methods: Logistic regression analysis was conducted on a cohort of 465 CHB patients from two hospitals to determine whether NAFLD was a risk factor for adverse reactions to NAs. CHB patients were followed up for more than 28 months after initial antiviral treatment, and further validation was performed using different viral load populations.
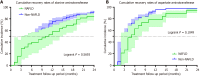
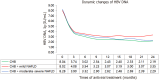
Results: NAFLD was identified as an independent risk factor for partial virological response following antiviral therapy with NAs (odds ratio = 1.777, P = 0.017). In our subsequent analysis focusing on CHB patients with high viral load, the NAFLD group exhibited significantly longer virus shedding time and lower proportion of the complete virological response compared with the non-NAFLD group (16.8 ± 6.1 vs 13.0 ± 6.8, P < 0.05). During the 24-month period of antiviral treatment with NAs, hepatitis B virus (HBV) DNA levels decreased slowly in the NAFLD group, and the negative conversion rate of HBV was notably lower than that observed in non-NAFLD group (P = 0.001). Similar results were obtained when analyzing patients with low baseline HBV viral load within the NAFLD group.
Conclusion: Coexistence of NAFLD may diminish virological response among CHB patients receiving antiviral treatment with NAs.
Keywords: Antiviral therapy; Chronic hepatitis B; Nonalcoholic fatty liver disease; Nucleoside analogues; Virological response.
©The Author(s) 2024. Published by Baishideng Publishing Group Inc. All rights reserved.
Conflict of interest statement
Conflict-of-interest statement: The authors declare that they have no conflict of interest.
Figures


References
-
- Cai J, Zhang XJ, Li H. Progress and challenges in the prevention and control of nonalcoholic fatty liver disease. Med Res Rev. 2019;39:328–348. - PubMed
-
- World Health Organization. Global hepatitis report 2017. Geneva: World Health Organization; 2017. Available from: http://www.who.int/hepatitis/publications/global-hepatitis-report2017/en/
-
- Choi HSJ, Brouwer WP, Zanjir WMR, de Man RA, Feld JJ, Hansen BE, Janssen HLA, Patel K. Nonalcoholic Steatohepatitis Is Associated With Liver-Related Outcomes and All-Cause Mortality in Chronic Hepatitis B. Hepatology. 2020;71:539–548. - PubMed
-
- Machado MV, Oliveira AG, Cortez-Pinto H. Hepatic steatosis in hepatitis B virus infected patients: meta-analysis of risk factors and comparison with hepatitis C infected patients. J Gastroenterol Hepatol. 2011;26:1361–1367. - PubMed
LinkOut - more resources
Full Text Sources

