Cardiac cells and mesenchymal stem cells derived extracellular vesicles: a potential therapeutic strategy for myocardial infarction
- PMID: 39744211
- PMCID: PMC11688320
- DOI: 10.3389/fcvm.2024.1493290
Cardiac cells and mesenchymal stem cells derived extracellular vesicles: a potential therapeutic strategy for myocardial infarction
Abstract
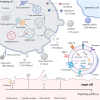
Despite improvements in clinical outcomes of acute myocardial infarction (AMI), mortality rates remain high, indicating the need for further understanding of the pathogenesis and developing more effective cardiac protection strategies. Extracellular vesicles (EVs) carry proteins and noncoding RNAs (ncRNAs) derived from different cardiac cell populations, mainly including cardiomyocytes, endothelial cells, endothelial progenitor cells, cardiac progenitor cells, cardiosphere-derived cells, immune cells, fibroblasts and cardiac telocytes have vital roles under both physiological and pathological process such as myocardial infarction (MI). The content of EVs can also indicate the status of their parental cells and serve as a biomarker for monitoring the risk of cardiac injury. Examining these vesicles can offer fresh perspectives on the development of MI and assist in creating innovative treatments. Additionally, mesenchymal stem cells (MSCs) (MSC-EVs) derived EVs have been shown to have significant potential in cardiac regeneration. In this review, we will discuss the current understanding of the role of EVs in cardiac communication, with a focus on the perspectives of EVs from various cardiac cells and MSCs for their potential uses as cardiac therapies after MI.
Keywords: cardiac cells; cell-cell communication; extracellular vesicles; mesenchymal stem cells; myocardial infarction.
© 2024 Qin, Wang, Pu and Hu.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
Extracellular vesicle-loaded hydrogels for tissue repair and regeneration.Mater Today Bio. 2022 Dec 21;18:100522. doi: 10.1016/j.mtbio.2022.100522. eCollection 2023 Feb. Mater Today Bio. 2022. PMID: 36593913 Free PMC article. Review.
-
Protective Effects of MicroRNA-200b-3p Encapsulated by Mesenchymal Stem Cells-Secreted Extracellular Vesicles in Myocardial Infarction Via Regulating BCL2L11.J Am Heart Assoc. 2022 Jun 21;11(12):e024330. doi: 10.1161/JAHA.121.024330. Epub 2022 Jun 14. J Am Heart Assoc. 2022. PMID: 35699193 Free PMC article.
-
The Vital Roles of Mesenchymal Stem Cells and the Derived Extracellular Vesicles in Promoting Angiogenesis After Acute Myocardial Infarction.Stem Cells Dev. 2021 Jun 1;30(11):561-577. doi: 10.1089/scd.2021.0006. Epub 2021 Apr 30. Stem Cells Dev. 2021. PMID: 33752473 Review.
-
Extracellular vesicles from dental pulp mesenchymal stem cells modulate macrophage phenotype during acute and chronic cardiac inflammation in athymic nude rats with myocardial infarction.Inflamm Regen. 2024 May 28;44(1):25. doi: 10.1186/s41232-024-00340-7. Inflamm Regen. 2024. PMID: 38807194 Free PMC article.
-
Extracellular vesicles derived from human bone marrow mesenchymal stem cells promote angiogenesis in a rat myocardial infarction model.J Mol Med (Berl). 2014 Apr;92(4):387-97. doi: 10.1007/s00109-013-1110-5. Epub 2013 Dec 14. J Mol Med (Berl). 2014. PMID: 24337504
Cited by
-
Elucidating the preventive and therapeutic effects of cardiac telocytes paracrine microRNAs on ischemic heart disease.Front Cardiovasc Med. 2025 Apr 1;12:1540051. doi: 10.3389/fcvm.2025.1540051. eCollection 2025. Front Cardiovasc Med. 2025. PMID: 40236257 Free PMC article.
References
-
- Christensen DM, Schjerning A-M, Smedegaard L, Charlot MG, Ravn PB, Ruwald AC, et al. Long-term mortality, cardiovascular events, and bleeding in stable patients 1 year after myocardial infarction: a Danish nationwide study. Eur Heart J. (2023) 44(6):488–98. 10.1093/eurheartj/ehac667 - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources

