Revolutionizing drug delivery: low-intensity pulsed ultrasound (LIPUS)-driven deep penetration into hypoxic tumor microenvironments of cholangiocarcinoma
- PMID: 39744223
- PMCID: PMC11667228
- DOI: 10.7150/thno.99981
Revolutionizing drug delivery: low-intensity pulsed ultrasound (LIPUS)-driven deep penetration into hypoxic tumor microenvironments of cholangiocarcinoma
Abstract
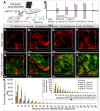
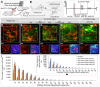
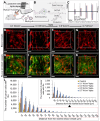
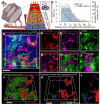
Background: Hypoxia is a major obstacle in the treatment of solid tumors because it causes immune escape and therapeutic resistance. Drug penetration into the hypoxic regions of tumor microenvironment (TME) is extremely limited. This study proposes using the unidirectional fluid flow property of low-intensity pulsed ultrasound (LIPUS) to overcome drug penetration limitations in the TME. LIPUS is gaining attention as a therapeutic modality for cancer owing to its safety and efficacy. Methods: LIPUS parameters, such as the intensity, duty cycle (DC), and duration, were optimized to enhance drug delivery into the hypoxic regions of the TME in cholangiocarcinoma (CCA). Transparent tumor imaging using the tissue optical clearing method (CLARITY) enabled 3D visualization and quantitative assessment of drug delivery and therapeutic efficacy in relation to blood vessels in an intact tumor at the micrometer level. The antitumor efficacy of LIPUS-assisted chemotherapy was evaluated in a CCA xenograft mouse model. Results: LIPUS significantly enhanced drug delivery efficacy into the hypoxic region of the TME in CCA. Under optimal conditions, i.e., a DC of 45% and a spatial-peak temporal-average intensity (Ispta) of 0.5 W/cm², drug penetration, including liposomal nanoparticles and chemotherapeutic agents gemcitabine and cisplatin, was improved by approximately 1.8-fold, resulting in a fivefold increase in apoptotic cancer cell death and a significant reduction in CCA growth. Notably, drug penetration and efficacy were more significantly affected by DC compared to the spatial-peak pulse-average intensity (Isppa). The efficacy saturated at Ispta values above 0.5 W/cm² under a 45% DC. Furthermore, we confirm that LIPUS induces non-thermal effects without causing cell damage, ensuring biosafety. These findings highlight the potential of LIPUS as a non-invasive strategy for treating hypoxic tumors. Conclusion: LIPUS adjuvant therapy promises improved cancer treatment outcomes and offers a safe and innovative therapeutic strategy for CCA and other hypoxic tumors.
Keywords: Cancer imaging; Chemotherapy; LIPUS; Liposome; Unidirectional fluid flow.
© The author(s).
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures





Similar articles
-
Atorvastatin Augments Gemcitabine-Mediated Anti-Cancer Effects by Inhibiting Yes-Associated Protein in Human Cholangiocarcinoma Cells.Int J Mol Sci. 2020 Oct 14;21(20):7588. doi: 10.3390/ijms21207588. Int J Mol Sci. 2020. PMID: 33066548 Free PMC article.
-
Enhanced expression of ten-eleven translocation 1 reverses gemcitabine resistance in cholangiocarcinoma accompanied by a reduction in P-glycoprotein expression.Cancer Med. 2019 Mar;8(3):990-1003. doi: 10.1002/cam4.1983. Epub 2019 Feb 19. Cancer Med. 2019. PMID: 30784212 Free PMC article.
-
Gemcitabine-loaded DSPE-PEG-PheoA liposome as a photomediated immune modulator for cholangiocarcinoma treatment.Biomaterials. 2018 Nov;183:139-150. doi: 10.1016/j.biomaterials.2018.08.052. Epub 2018 Aug 23. Biomaterials. 2018. PMID: 30170256
-
Telotristat ethyl, a tryptophan hydroxylase inhibitor, enhances antitumor efficacy of standard chemotherapy in preclinical cholangiocarcinoma models.J Cell Mol Med. 2024 Sep;28(17):e18585. doi: 10.1111/jcmm.18585. J Cell Mol Med. 2024. PMID: 39223878 Free PMC article.
-
Attenuation of orthodontically induced inflammatory root resorption by using low-intensity pulsed ultrasound as a therapeutic modality- a systematic review.BMC Oral Health. 2024 Jan 10;24(1):67. doi: 10.1186/s12903-023-03741-1. BMC Oral Health. 2024. PMID: 38200481 Free PMC article.
Cited by
-
Cardiac rehabilitation in porcine models: Advances in therapeutic strategies for ischemic heart disease.Zool Res. 2025 May 18;46(3):576-607. doi: 10.24272/j.issn.2095-8137.2024.387. Zool Res. 2025. PMID: 40343415 Free PMC article. Review.
References
-
- Seidi K, Neubauer HA, Moriggl R, Jahanban-Esfahlan R, Javaheri T. Tumor target amplification: Implications for nano drug delivery systems. J Control Release. 2018;275:142–61. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

