CXCR4-directed endoradiotherapy with [177Lu]Pentixather added to total body irradiation for myeloablative conditioning in patients with relapsed/refractory acute myeloid leukemia
- PMID: 39744224
- PMCID: PMC11667225
- DOI: 10.7150/thno.101215
CXCR4-directed endoradiotherapy with [177Lu]Pentixather added to total body irradiation for myeloablative conditioning in patients with relapsed/refractory acute myeloid leukemia
Abstract
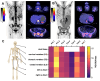
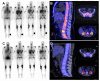
Rationale: Despite recent advances in the targeted therapy of AML, the disease continues to have a poor prognosis. Allogeneic hematopoietic stem cell transplantation (alloSCT) remains to be the curative therapy option for fit patients with high-risk disease. Especially patients with relapsed or refractory (r/r) AML continue to have poor outcomes. Myeloablative total body irradiation (TBI) based conditioning can be used in AML patients refractory to multiple lines of standard therapy, but the optimal conditioning regimen remains unclear for patients considered to be chemotherapy- refractory. Feasibility of C-X-C-motif chemokine receptor 4 (CXCR4)-directed endoradiotherapy (ERT) has previously been demonstrated in AML patients with CXCR4 expression on leukemic blasts. Methods: Here, we report on a small cohort of seven AML patients refractory to multiple lines (range 3-7) of therapy, who received CXCR4-directed ERT with [177Lu]Pentixather in combination with TBI and chemotherapy prior to alloSCT. We report outcomes with a focus on toxicity, engraftment, the impact on the bone marrow (BM) niche and efficacy. Results: In this intensively pre-treated group of patients, promising response (6 out of 7 patients) and engraftment (6 out of 7 patients) rates were observed. Histopathological analysis showed that niche compartments are spared and allow for engraftment to occur despite the combined ERT and TBI conditioning. Conclusion: To the best of our knowledge, we report on the first seven patients who received CXCR4-directed ERT in sequential combination with TBI and chemotherapy, providing an effective, individualized conditioning regimen for intensively pre-treated r/r AML patients.
Keywords: CXCR4; acute myeloid leukemia; allogeneic stem cell transplantation; conditioning regimens; endoradiotherapy.
© The author(s).
Conflict of interest statement
Competing Interests: WW reports Research Support from Siemens Healthineers, Blue Earth Diagnostics, ITM, Novartis, Pentixapharm, Ratio Therapeutics, Rayze Bio, Trimt, Roche. Employee/consultant/stock holder from Immu-Veo, ITM, Novartis, Rayze Bio, Roche. Honoraria from Siemens Healthineers and Scientific advisory board from Immune-Image, Immu-Veo. FB received honoraria from BMS and Janssen. ME reports fees from Blue Earth Diagnostics Ltd. (consultant, research funding), Novartis/AAA (consultant, speaker), Telix (consultant), Bayer (consultant, research funding), RayzeBio (consultant), Point Biopharma (consultant), Eckert-Ziegler (speaker), ABX GmbH (speaker) and Janssen Pharmaceuticals (consultant, speakers bureau), Parexel (image review) and Bioclinica (image review) outside the submitted work and a patent application for rhPSMA. He and other inventors are entitled to royalties on sales of POSLUMA®. PH reports personal fees and non-financial funding from Pentixapharm and personal fees from Immedica.
Figures

References
-
- DiNardo CD, Jonas BA, Pullarkat V, Thirman MJ, Garcia JS, Wei AH. et al. Azacitidine and Venetoclax in Previously Untreated Acute Myeloid Leukemia. N Engl J Med. 2020;383:617–29. - PubMed
-
- Montesinos P, Recher C, Vives S, Zarzycka E, Wang J, Bertani G. et al. Ivosidenib and Azacitidine in IDH1-Mutated Acute Myeloid Leukemia. N Engl J Med. 2022;386:1519–31. - PubMed
-
- Döhner H, Wei AH, Appelbaum FR, Craddock C, DiNardo CD, Dombret H. et al. Diagnosis and management of AML in adults: 2022 recommendations from an international expert panel on behalf of the ELN. Blood. 2022;140:1345–77. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

