Optical coherence tomography (OCT) and OCT angiography: Technological development and applications in brain science
- PMID: 39744229
- PMCID: PMC11667229
- DOI: 10.7150/thno.97192
Optical coherence tomography (OCT) and OCT angiography: Technological development and applications in brain science
Abstract
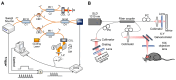
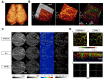
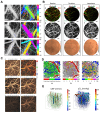
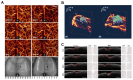
Brain diseases are a leading cause of disability and death worldwide. Early detection can lead to earlier intervention and better outcomes for patients. In recent years, optical coherence tomography (OCT) and OCT angiography (OCTA) imaging have been widely used in stroke, traumatic brain injury (TBI), and brain cancer due to their advantages of in vivo, unlabeled, and high-resolution 3D microvessel imaging at the capillary resolution level. This review summarizes recent advances and challenges in living brain imaging using OCT/OCTA, including technique modality, types of diseases, and theoretical approach. Although there may still be many limitations, with the development of lasers and the advances in artificial intelligence are expected to enable accurate detection of deep cerebral hemodynamics and guide intraoperative tumor resection in vivo in the future.
Keywords: brain cancer; ischemic stroke; optical coherence tomography; traumatic brain injury.
© The author(s).
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures





References
-
- Zwanenburg JJ, Hendrikse J, Takahara T, Visser F, Luijten PR. MR angiography of the cerebral perforating arteries with magnetization prepared anatomical reference at 7 T: comparison with time-of-flight. J Magn Reson Imaging. 2008;28(6):1519–1526. - PubMed
-
- Villablanca JP, Nael K, Habibi R, Nael A, Laub G, Finn JP. 3 T contrast-enhanced magnetic resonance angiography for evaluation of the intracranial arteries: comparison with time-of-flight magnetic resonance angiography and multislice computed tomography angiography. Invest Radiol. 2006;41(11):799–805. - PubMed
-
- Hori M, Shiraga N, Watanabe Y, Aoki S, Isono S, Yui M. et al. Time-resolved three-dimensional magnetic resonance digital subtraction angiography without contrast material in the brain: Initial investigation. J Magn Reson Imaging. 2009;30(1):214–218. - PubMed
-
- de Havenon A, Mossa-Basha M, Shah L, Kim SE, Park M, Parker D. et al. High-resolution vessel wall MRI for the evaluation of intracranial atherosclerotic disease. Neuroradiology. 2017;59(12):1193–1202. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

