iRGD-TRP-PK1-modified red blood cell membrane vesicles as a new chemotherapeutic drug delivery and targeting system in head and neck cancer
- PMID: 39744235
- PMCID: PMC11667238
- DOI: 10.7150/thno.99481
iRGD-TRP-PK1-modified red blood cell membrane vesicles as a new chemotherapeutic drug delivery and targeting system in head and neck cancer
Abstract
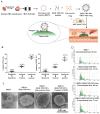
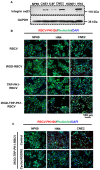
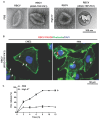
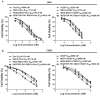
Background: Chemotherapy is essential for treating tumors, including head and neck cancer (HNC). However, the toxic side effects of chemotherapeutic drugs limit their widespread use. Therefore, a targeted delivery system that can transport the drug to the pathological site while minimizing damage to healthy tissues is urgently needed. Methods: Application of animal imaging, flow cytometry, fluorescence staining, cell activity assay, transmission electron microscopy, western blotting and immunohistochemistry to evaluate the targeting and killing effects of internalizing RGD peptide (iRGD)-transient receptor potential (TRP)-PK1-modified red blood cell vesicles (RBCVs) on HNC cells in vitro and in vivo. Results: TRP-PK1 was ligated to iRGD, enabling autonomous insertion into the lipid bilayer. Additionally, RBCVs were labeled with iRGD-TRP-PK1 to achieve tumor targeting. Based on the self-assembly capability of TRP-PK1 to form a "leakage potassium" channel on the biofilm, RBCVs were fragmented within the high-potassium (K+) environment inside tumor cells. This fragmentation facilitated the release of the drug loaded onto the RBCVs. Conclusion: The advantageous properties of TRP-PK1 are utilized in our design, resulting in a cost-effective and straightforward approach to drug delivery and release. Ultimately, the objective of suppressing tumor growth while minimizing side effects was accomplished by iRGD-TRP-PK1-modified RBCVs in our study. These findings provide novel insights into the enhancement of targeted delivery systems and present promising avenues for the treatment of HNC.
Keywords: Drug Delivery; Head and Neck Cancer; Red Blood Cell Membrane Vesicles; Targeting System; iRGD-TRP-PK1.
© The author(s).
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures





Similar articles
-
Lipid insertion enables targeted functionalization of paclitaxel-loaded erythrocyte membrane nanosystem by tumor-penetrating bispecific recombinant protein.Int J Nanomedicine. 2018 Sep 11;13:5347-5359. doi: 10.2147/IJN.S165109. eCollection 2018. Int J Nanomedicine. 2018. PMID: 30254439 Free PMC article.
-
Co-Administration Of iRGD Enhances Tumor-Targeted Delivery And Anti-Tumor Effects Of Paclitaxel-Loaded PLGA Nanoparticles For Colorectal Cancer Treatment.Int J Nanomedicine. 2019 Nov 1;14:8543-8560. doi: 10.2147/IJN.S219820. eCollection 2019. Int J Nanomedicine. 2019. PMID: 31802868 Free PMC article.
-
iRGD-Guided Silica/Gold Nanoparticles for Efficient Tumor-Targeting and Enhancing Antitumor Efficacy Against Breast Cancer.Int J Nanomedicine. 2024 Aug 12;19:8237-8251. doi: 10.2147/IJN.S474135. eCollection 2024. Int J Nanomedicine. 2024. PMID: 39157735 Free PMC article.
-
Recent advances in the tumor-penetrating peptide internalizing RGD for cancer treatment and diagnosis.Drug Dev Res. 2023 Jun;84(4):654-670. doi: 10.1002/ddr.22056. Epub 2023 Apr 5. Drug Dev Res. 2023. PMID: 36959702 Review.
-
Enhancing Tumor Targeted Therapy: The Role of iRGD Peptide in Advanced Drug Delivery Systems.Cancers (Basel). 2024 Nov 8;16(22):3768. doi: 10.3390/cancers16223768. Cancers (Basel). 2024. PMID: 39594723 Free PMC article. Review.
Cited by
-
Engineered endoplasmic reticulum-targeting nanodrugs with Piezo1 inhibition and promotion of cell uptake for subarachnoid hemorrhage inflammation repair.J Nanobiotechnology. 2025 Apr 5;23(1):274. doi: 10.1186/s12951-025-03305-1. J Nanobiotechnology. 2025. PMID: 40186204 Free PMC article.
-
Peptide based vesicles for cancer immunotherapy: design, construction and applications.Front Immunol. 2025 May 27;16:1609162. doi: 10.3389/fimmu.2025.1609162. eCollection 2025. Front Immunol. 2025. PMID: 40496863 Free PMC article. Review.
References
-
- Koh J, Walsh P, D'Costa I, Bhatti O. Head and neck squamous cell carcinoma survivorship care. Aust J Gen Pract. 2019;48:846–8. - PubMed
-
- Neville BW, Day TA. Oral cancer and precancerous lesions. CA Cancer J Clin. 2002;52:195–215. - PubMed
-
- Ove R, Nabell LM. Induction chemotherapy for head and neck cancer: is there still a role? Future Oncol. 2016;12:1595–608. - PubMed
-
- Seyyednia E, Oroojalian F, Baradaran B, Mojarrad JS, Mokhtarzadeh A, Valizadeh H. Nanoparticles modified with vasculature-homing peptides for targeted cancer therapy and angiogenesis imaging. J Control Release. 2021;338:367–93. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

