A Prospective, Noninterventional Study to Evaluate the Impact of Emicizumab in the Management of Hemophilia A
- PMID: 39744287
- PMCID: PMC11688609
- DOI: 10.7759/cureus.74948
A Prospective, Noninterventional Study to Evaluate the Impact of Emicizumab in the Management of Hemophilia A
Abstract
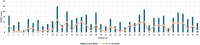
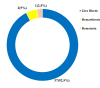
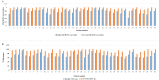
Background and objective Hemophilia A (HA) is a genetic bleeding disorder caused by a lack of factor VIII (FVIII) and is associated with frequent bleeding and joint damage. Traditional intravenous treatments for this condition are cumbersome and can lead to complications. Emicizumab, a bispecific monoclonal antibody, offers a promising subcutaneous alternative with potential safety and efficacy-related benefits. This study aimed to evaluate the impact of emicizumab prophylaxis on bleeding rates, joint health, functional activity, and quality of life (QoL) in patients with congenital HA. Methods A noninterventional, prospective observational study was conducted at the Assam Medical College, a tertiary care center in northeastern India, involving 40 patients with HA (PwHA), who were either on FVIII therapy or newly on emicizumab. Emicizumab was given subcutaneously at 3 mg/kg weekly for the first month, followed by 6 mg/kg every four weeks. Endpoints included changes in annual bleeding rate (ABR), Hemophilia Joint Health Score (HJHS), Functional Independence Score in Hemophilia (FISH), and QoL via European Quality of Life 5-Dimensions 5-Levels (EQ-5D-5L) and visual analog scale (VAS) scores at 24 weeks. Results At 24 weeks, HJHS improved from 12.8 to 4.8 (p<0.001), FISH from 27.5 to 30.6 (p<0.001), and ABR decreased from 11.36 to 0.195. Quality of life scores also improved (EQ-5D-5L index from 0.79 to 0.96, VAS from 72.18 to 92.75, both p<0.001). All 30 patients with target joints had resolved bleeds, and adherence to emicizumab was 100%. Conclusions Based on our findings, emicizumab significantly reduces bleeding, enhances joint health, and improves the quality of life in PwHA. It is associated with high adherence, suggesting its feasibility as a treatment, especially in resource-limited settings. However, long-term studies are needed to validate these results.
Keywords: bleeding rate; coagulation factor viii; emicizumab; functional independence; hemophilia a; quality of life; visual analog score.
Copyright © 2024, Dutta et al.
Conflict of interest statement
Human subjects: Consent for treatment and open access publication was obtained or waived by all participants in this study. Institutional Ethics Committee (H) Assam Medical College, Dibrugarh 786002 (Reg. no. ECR/636/Inst/AS/2014) issued approval 2023/AMC/EC/10795. Animal subjects: All authors have confirmed that this study did not involve animal subjects or tissue. Conflicts of interest: In compliance with the ICMJE uniform disclosure form, all authors declare the following: Payment/services info: All authors have declared that no financial support was received from any organization for the submitted work. Financial relationships: All authors have declared that they have no financial relationships at present or within the previous three years with any organizations that might have an interest in the submitted work. Other relationships: All authors have declared that there are no other relationships or activities that could appear to have influenced the submitted work.
Figures





References
-
- The World Federation of Hemophilia Annual Global Survey 1999-2018. Stonebraker JS, Bolton-Maggs PH, Brooker M, et al. Haemophilia. 2020;26:591–600. - PubMed
-
- World Federation of Hemophilia Report on Annual Global Survey. [ Nov; 2024 ]. 2023. https://www1.wfh.org/publications/files/pdf-2525.pdf https://www1.wfh.org/publications/files/pdf-2525.pdf
-
- World Federation of Hemophilia: World Bleeding Disorders Registry Data Dashboard. [ Nov; 2024 ]. 2023. https://wfh.org/research-and-data-collection/world-bleeding-disorders-re... https://wfh.org/research-and-data-collection/world-bleeding-disorders-re...
LinkOut - more resources
Full Text Sources
