Spinal cord injury: pathophysiology, possible treatments and the role of the gut microbiota
- PMID: 39744391
- PMCID: PMC11688470
- DOI: 10.3389/fmicb.2024.1490855
Spinal cord injury: pathophysiology, possible treatments and the role of the gut microbiota
Abstract
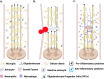
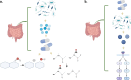
Spinal cord injury (SCI) is a devastating pathological state causing motor, sensory, and autonomic dysfunction. To date, SCI remains without viable treatment for its patients. After the injury, molecular events centered at the lesion epicenter create a non-permissive environment for cell survival and regeneration. This newly hostile setting is characterized by necrosis, inflammation, demyelination, axotomy, apoptosis, and gliosis, among other events that limit locomotor recovery. This review provides an overview of the pathophysiology of SCI, highlighting the potential role of the gut microbiota in modulating the inflammatory response and influencing neurological recovery following trauma to the spinal cord. Emphasis on the bidirectional communication between the gut and central nervous system, known as the gut-brain axis is given. After trauma, the gut-brain/spinal cord axis promotes the production of pro-inflammatory metabolites that provide a non-permissive environment for cell survival and locomotor recovery. Therefore, any possible pharmacological treatment, including antibiotics and painkillers, must consider their effects on microbiome dysbiosis to promote cell survival, regeneration, and behavioral improvement. Overall, this review provides valuable insights into the pathophysiology of SCI and the evolving understanding of the role of the gut microbiota in SCI, with implications for future research and clinical practice.
Keywords: dysbiosis; gut microbiome; gut-brain axis; neurodegenerative; spinal cord trauma.
Copyright © 2024 Pagan-Rivera, Ocasio-Rivera, Godoy-Vitorino and Miranda.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

