Advanced Lung-on-a-Chip Technology: Mimicking the Complex Human Lung Microenvironment
- PMID: 39744426
- PMCID: PMC11667821
- DOI: 10.7150/ijbs.105702
Advanced Lung-on-a-Chip Technology: Mimicking the Complex Human Lung Microenvironment
Abstract
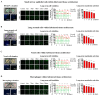
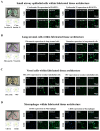
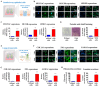
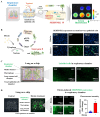
Intricate crosstalk among various lung cell types is crucial for orchestrating diverse physiological processes. Traditional two-dimensional and recent three-dimensional (3D) assay platforms fail to precisely replicate these complex communications. Many in vitro lung models do not effectively reflect the multicellular complexity of lung tissue. Here, we fabricated an advanced multicellular 3D lung-on-a-chip system that properly replicates the dynamic pulmonary microenvironment and its intricate microarchitecture. Diverse lung cells were incorporated into a microstructure formed from a mixture of natural polymers, including collagen and hyaluronic acid, and blood coagulation factors acting as natural crosslinking agents. The system accurately reflects the complex 3D architecture of the lung. Biomarkers demonstrate more rapid and sensitive responses to toxic substances than functional indicators, such as cell proliferation and apoptosis. SERPINB2 was identified as a biomarker of lung toxicity; it was activated in small airway epithelial cells exposed to various toxic substances. We then developed a fluorescence-linked toxicity biomarker screening platform that enables both intuitive and quantitative evaluation of lung toxicity by measuring the converted fluorescent signal strength. This fluorescent tagging system was incorporated into small airway epithelial cells within a fabricated chip platform; enabling lung-on-a-chip enabled evaluation of the lung toxicity of prospective drug candidates.
Keywords: Fluorescence Screening; Lung Toxicity; Lung-on-a-Chip; Natural Polymers; SERPINB2.
© The author(s).
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures





Similar articles
-
Development of Advanced Oral-on-a-Chip: Replicating the Intricate Human Oral Microenvironment.Int J Biol Sci. 2024 Oct 28;20(15):5888-5909. doi: 10.7150/ijbs.104351. eCollection 2024. Int J Biol Sci. 2024. PMID: 39664582 Free PMC article.
-
Development of a novel testis-on-a-chip that demonstrates reciprocal crosstalk between Sertoli and Leydig cells in testicular tissue.Exp Mol Med. 2024 Jul;56(7):1591-1605. doi: 10.1038/s12276-024-01258-3. Epub 2024 Jul 1. Exp Mol Med. 2024. PMID: 38945952 Free PMC article.
-
Microfluidic lung airway-on-a-chip with arrayable suspended gels for studying epithelial and smooth muscle cell interactions.Lab Chip. 2018 May 1;18(9):1298-1309. doi: 10.1039/c7lc01357d. Lab Chip. 2018. PMID: 29651473
-
Recent advances in lung-on-a-chip models.Drug Discov Today. 2022 Sep;27(9):2593-2602. doi: 10.1016/j.drudis.2022.06.004. Epub 2022 Jun 18. Drug Discov Today. 2022. PMID: 35724916 Review.
-
Organ-on-a-chip: Quo vademus? Applications and regulatory status.Colloids Surf B Biointerfaces. 2025 May;249:114507. doi: 10.1016/j.colsurfb.2025.114507. Epub 2025 Jan 8. Colloids Surf B Biointerfaces. 2025. PMID: 39826309 Review.
References
-
- Zamprogno P, Schulte J, Ferrari D, Rechberger K, Sengupta A, van Os L. et al. Lung-on-a-Chip Models of the Lung Parenchyma. Adv Exp Med Biol. 2023;1413:191–211. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

