Ganglioside GA2-mediated caspase-11 activation drives macrophage pyroptosis aggravating intimal hyperplasia after arterial injury
- PMID: 39744431
- PMCID: PMC11667815
- DOI: 10.7150/ijbs.97106
Ganglioside GA2-mediated caspase-11 activation drives macrophage pyroptosis aggravating intimal hyperplasia after arterial injury
Abstract
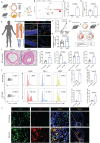
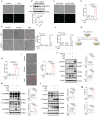
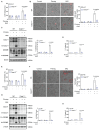
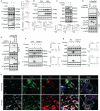
Intimal hyperplasia (IH) remains a significant clinical problem, causing vascular intervention failure. This study aimed to elucidate whether gangliosides GA2 accumulated in atherosclerotic mouse aortae and plasma promote the development of IH. We identified that GA2 was remarkably accumulated in both artery and plasma of atherosclerotic patients and mice. Injected GA2 exacerbated IH and mainly co-stained with macrophages after mouse carotid arterial injury model. Intracellular GA2 induced pyroptosis accompanying the IL-1α release, which was blocked by caspase-11 knockout. Mechanistically, GA2 directly activated caspase-4 as a new ligand. And then, activated caspase-4/11 combined and cleaved BID, promoting the cytochrome C release to cytoplasm, which derived gasdermin E-medicated pyroptosis through activation of caspase-9-caspase-3 pathway. Mice transplanted with caspase-11 deficient bone marrow or mice with caspase-11 knockdown in macrophages exhibited an improvement of the IH aggravated by GA2. These findings suggest GA2-mediated caspase-4/11 activation drives macrophage pyroptosis, contributing to IH. Our results provide a potential diagnostic and therapeutic target in IH.
© The author(s).
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures




Similar articles
-
IL-27 aggravates acute hepatic injury by promoting macrophage M1 polarization to induce Caspase-11 mediated Pyroptosis in vitro and in vivo.Cytokine. 2025 Apr;188:156881. doi: 10.1016/j.cyto.2025.156881. Epub 2025 Feb 5. Cytokine. 2025. PMID: 39913960
-
HMGB1-Driven Inflammation and Intimal Hyperplasia After Arterial Injury Involves Cell-Specific Actions Mediated by TLR4.Arterioscler Thromb Vasc Biol. 2015 Dec;35(12):2579-93. doi: 10.1161/ATVBAHA.115.305789. Epub 2015 Oct 29. Arterioscler Thromb Vasc Biol. 2015. PMID: 26515416 Free PMC article.
-
Mechanical Ventilation Exacerbates Poly (I:C) Induced Acute Lung Injury: Central Role for Caspase-11 and Gut-Lung Axis.Front Immunol. 2021 Jul 19;12:693874. doi: 10.3389/fimmu.2021.693874. eCollection 2021. Front Immunol. 2021. PMID: 34349759 Free PMC article.
-
Caspase-4/11-Mediated Pulmonary Artery Endothelial Cell Pyroptosis Contributes to Pulmonary Arterial Hypertension.Hypertension. 2022 Mar;79(3):536-548. doi: 10.1161/HYPERTENSIONAHA.121.17868. Epub 2022 Jan 5. Hypertension. 2022. PMID: 34984912
-
Caspase-11/4 and gasdermin D-mediated pyroptosis contributes to podocyte injury in mouse diabetic nephropathy.Acta Pharmacol Sin. 2021 Jun;42(6):954-963. doi: 10.1038/s41401-020-00525-z. Epub 2020 Sep 23. Acta Pharmacol Sin. 2021. PMID: 32968210 Free PMC article.
Cited by
-
The role of stem cell-derived exosomes in regulating pyroptosis for disease therapy.Stem Cell Res Ther. 2025 Jul 18;16(1):386. doi: 10.1186/s13287-025-04519-8. Stem Cell Res Ther. 2025. PMID: 40682142 Free PMC article. Review.
-
Post-translational modifications orchestrate mTOR-driven cell death in cardiovascular disease.Front Cardiovasc Med. 2025 Jul 15;12:1620669. doi: 10.3389/fcvm.2025.1620669. eCollection 2025. Front Cardiovasc Med. 2025. PMID: 40734978 Free PMC article. Review.
References
-
- Breiden B, Sandhoff K. Lysosomal Glycosphingolipid Storage Diseases. Annual review of biochemistry. 2019;88:461–85. - PubMed
-
- Mukhin DN, Prokazova NV, Bergelson LD, Orekhov AN. Ganglioside content and composition of cells from normal and atherosclerotic human aorta. Atherosclerosis. 1989;78:39–45. - PubMed
-
- Mukhin DN, Chao FF, Kruth HS. Glycosphingolipid accumulation in the aortic wall is another feature of human atherosclerosis. Arteriosclerosis, thrombosis, and vascular biology. 1995;15:1607–15. - PubMed
-
- Garner B, Priestman DA, Stocker R, Harvey DJ, Butters TD, Platt FM. Increased glycosphingolipid levels in serum and aortae of apolipoprotein E gene knockout mice. Journal of lipid research. 2002;43:205–14. - PubMed
-
- Galis ZS, Muszynski M, Sukhova GK, Simon-Morrissey E, Libby P. Enhanced expression of vascular matrix metalloproteinases induced in vitro by cytokines and in regions of human atherosclerotic lesions. Annals of the New York Academy of Sciences. 1995;748:501–7. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

