Artemisinin Suppressed Melanoma Recurrence and Metastasis after Radical Surgery through the KIT/PI3K/AKT Pathway
- PMID: 39744440
- PMCID: PMC11667813
- DOI: 10.7150/ijbs.97341
Artemisinin Suppressed Melanoma Recurrence and Metastasis after Radical Surgery through the KIT/PI3K/AKT Pathway
Abstract
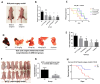
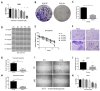
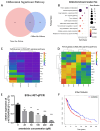
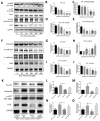
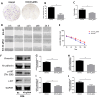
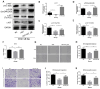
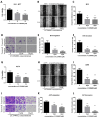
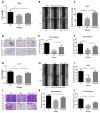
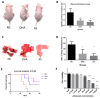
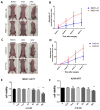
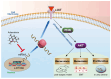
Cancer radical surgery is the primary treatment for melanoma, but almost all malignant melanoma patients get recurrence and metastasis after surgery and are eventually dead. This clinical dilemma appeals to better drugs for post-surgery therapy. Artemisinin is a safe and effective antimalarial drug used in the clinic for decades. However, no information is available regarding the effect of Artemisinins on melanoma recurrence and metastasis after tumor excision. In the present study, we established a post-surgery tumor model on balb/c nude mice, and we found that subclinical dosages of Artemisinin significantly blocked recurrence, metastasis, and extended survival time of mice after tumor excision. Similar results were obtained in the in vitro experiments with B16 and A375 cell lines. Further experiments confirmed that Artemisinin inhibits melanoma in vitro and in vivo after radical surgery by the c-KIT/PI3K/AKT signaling pathway. Our findings support the therapeutic potential of Artemisinin in malignant melanoma after surgery.
Keywords: AKT; artemisinin; c-KIT; melanoma; post-surgery model.
© The author(s).
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures

References
-
- Spagnolo F, Queirolo P. Upcoming strategies for the treatment of metastatic melanoma. Archives of dermatological research. 2012;304:177–84. - PubMed
-
- Schadendorf D, Fisher DE, Garbe C, Gershenwald JE, Grob JJ, Halpern A. et al. Melanoma. Nature reviews Disease primers. 2015;1:15003. - PubMed
-
- Bhave P, Haydon A. Treatment of High Risk Resected Melanoma in Australia: Current Landscape and Practises. The Australasian journal of dermatology. 2020;61:203–9. - PubMed
-
- Agaimy A, Specht K, Stoehr R, Lorey T, Märkl B, Niedobitek G. et al. Metastatic Malignant Melanoma With Complete Loss of Differentiation Markers (Undifferentiated/Dedifferentiated Melanoma): Analysis of 14 Patients Emphasizing Phenotypic Plasticity and the Value of Molecular Testing as Surrogate Diagnostic Marker. The American journal of surgical pathology. 2016;40:181–91. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

