Characteristics of patients with non-severe infections of different SARS-CoV-2 omicron subvariants in China
- PMID: 39744529
- PMCID: PMC11688270
- DOI: 10.3389/fmed.2024.1511227
Characteristics of patients with non-severe infections of different SARS-CoV-2 omicron subvariants in China
Abstract
Objective: The aim of this study was to explore the clinical characteristics of patients infected with different Omicron subvariants presenting non-severe disease, evaluate the safety and efficacy of Azvudine for treatment of COVID-19, in order to broaden understanding of Omicron subvariant infections.
Method: A total of 244 individuals with Omicron subvariant (BA.2.76, n = 158; BA.5.1, n = 86) were included in the study. Demographic, clinical, and laboratory data of the study participants were collected and analyzed.
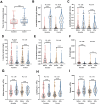
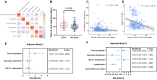
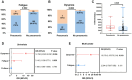
Result: Patients infected with BA.5.1 exhibited a higher incidence of clinical symptoms like fatigue (25.58% vs. 2.53%, p < 0.001), headache/dizziness (12.79% vs. 4.43%, p = 0.017), nausea/vomiting (10.47% vs. 1.27%, p = 0.002), viral loads and inflammatory factors, and shorter virus shedding time than those with BA.2.76. There are 28.1% patients reporting mild adverse events following Azvudine administration. After treatment, the levels of anti-SARS-CoV-2 IgG/IgM, white blood cell, and lymphocyte obviously increased, while C-reactive protein, procalcitonin, and D-dimer reduced. Azvudine speeded up the time for virus clearance compared to control treatment (10 vs. 11 days, p = 0.032). Low lymphocyte counts (odd ratio (OR) = 0.607, p = 0.001) and anti-SARS-CoV-2 IgG titer (OR = 0.990, p = 0.028) were the independent risk factors for long nucleic acid negativization duration after infection. Patients with pneumonia were often accompanied by dyspnea, fatigue and high level of D-dimer. Dyspnea (OR = 10.176, p = 0.019) could be used to identify the occurrence of pneumonia in patients infected with Omicron.
Conclusion: The study demonstrated the difference in clinical and laboratory parameters between patients infected with Omicron BA.2.76 and BA.5.1, as well as the safety and efficacy of Azvudine therapy. Our study linked patient manifestations to Omicron subvariant, treatment, and clinical outcomes, which is conducive to healthcare providers/policymakers to revise and implement appropriate countermeasures, facilitating appropriately advise for individuals with Omicron subvariant infections.
Keywords: Azvudine; BA.2.76; BA.5.1; clinical features; nucleic acid negativization; omicron subvariant; pneumonia.
Copyright © 2024 Yuan, Liu, Zhan, Wei, Zhang, Gao, Yan, Huang, Li and Dai.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- Adjei S, Hong K, Molinari NM, Bull-Otterson L, Ajani UA, Gundlapalli AV, et al. Mortality risk among patients hospitalized primarily for COVID-19 during the omicron and Delta variant pandemic periods - United States, April 2020-June 2022. MMWR Morb Mortal Wkly Rep. (2022) 71:1182–9. doi: 10.15585/mmwr.mm7137a4, PMID: - DOI - PMC - PubMed
-
- Ikuse T, Aizawa Y, Yamanaka T, Hasegawa S, Hayashi T, Kon M, et al. Comparison of clinical characteristics of children infected with coronavirus disease 2019 between omicron variant BA.5 and BA.1/BA.2 in Japan. Pediatr Infect Dis J. (2023) 42:503–9. doi: 10.1097/INF.0000000000003894, PMID: - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

