The effect of intraoperative radiotherapy in musculoskeletal malignancy: A population study from US SEER database
- PMID: 39744579
- PMCID: PMC11660128
- DOI: 10.7150/jca.100678
The effect of intraoperative radiotherapy in musculoskeletal malignancy: A population study from US SEER database
Abstract
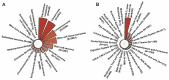
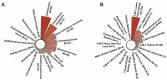
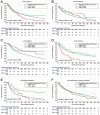
Objective: The aim of our study was to explore the effect of IORT on survival outcome of patients with musculoskeletal malignancy. The prognostic factors of patients with IORT treatment were also identified in this study. Methods: The retrospective analysis was conducted based on the Surveillance, Epidemiology, and End Results (SEER) database spanning from 2000 to 2020. The musculoskeletal malignancy patients who received both surgery and radiation therapy (RT) treatment were included into the study. Survival differences between groups were explored by Kaplan-Meier method and log-rank test. Potential prognostic factors of patients with IORT treatment were identified by Cox proportional hazards regression analysis. Results: A total of 24,297 patients were selected finally, including 23,877 cases with neoadjuvant/adjuvant RT alone, 190 cases with IORT alone, and other 230 cases received both neoadjuvant/adjuvant RT and IORT. The median survival time of these patients was 141.0 (95%CI: 101.1-180.9) months. Patients who received both IORT and neoadjuvant/adjuvant RT treatment presented the best survival outcome when compared with those underwent either IORT or neoadjuvant/adjuvant RT only. Further subgroup analyses verified the survival benefit of the combination of IORT and neoadjuvant/adjuvant RT in female patients with tumor located on limb and in patients who received the performance of chemotherapy. A series of variables, including age at diagnosis, gender, primary tumor site, tumor Grade, SEER stage, T stage, N stage, IORT only or the combination of IORT and neoadjuvant/adjuvant RT, the performance of chemotherapy, were identified as independent prognostic factors of patients with IORT treatment. Conclusions: The current study is distinguished by its large-scale analysis of the SEER database, encompassing a comprehensive cohort of musculoskeletal malignancy patients treated with IORT, as well as the rigorous subgroup analysis. We concluded that IORT during surgery procedure, accompanied with neoadjuvant/adjuvant RT, might confer a survival benefit for selected patients diagnosed with musculoskeletal malignancy.
Keywords: Bone Sarcoma; Intraoperative Radiotherapy; SEER Program; Soft Tissue Sarcoma; Survival Outcome.
© The author(s).
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures

References
-
- NCCN Guidelines Version 3. 2024. https://www.nccn.org/guidelines/guidelines-detail?category=1&id=1464.
-
- Strauss SJ, Frezza AM, Abecassis N. et al. Bone sarcomas: ESMO-EURACAN-GENTURIS-ERN PaedCan Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann Oncol. 2021;32:1520–1536. - PubMed
-
- Xu Y, Shi F, Zhang Y. et al. Twenty-year outcome of prevalence, incidence, mortality and survival rate in patients with malignant bone tumors. Int J Cancer. 2024;154:226–240. - PubMed
LinkOut - more resources
Full Text Sources

