Brain Network Alterations in Chronic Spinal Cord Injury: Multilayer Community Detection Approach
- PMID: 39744611
- PMCID: PMC11685503
- DOI: 10.1089/neur.2024.0098
Brain Network Alterations in Chronic Spinal Cord Injury: Multilayer Community Detection Approach
Abstract
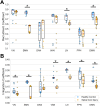
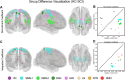
Neurological recovery in individuals with spinal cord injury (SCI) is multifaceted, involving mechanisms such as remyelination and perilesional spinal neuroplasticity, with cortical reorganization being one contributing factor. Cortical reorganization, in particular, can be evaluated through network (graph) analysis of interregional functional connectivity. This study aimed to investigate cortical reorganization patterns in persons with chronic SCI using a multilayer community detection approach on resting-state functional MRI data. Thirty-eight participants with chronic cervical or thoracic SCI and 32 matched healthy controls were examined. Significant alterations in brain community structures were observed in the SCI cohort, particularly within the sensorimotor network (SMN). Importantly, this revealed a pattern of segregation within the SMN, aligning with borders of representations of the upper and lower body and orofacial regions. The SCI cohort showed reduced recruitment and integration coefficients across multiple brain networks, indicating impaired internetwork communication that may underlie sensory and motor deficits in persons with SCI. These findings highlight the impact of SCI on brain connectivity and suggest potential compensatory mechanisms.
Keywords: cortical reorganization; functional connectivity; graph theory; mesoscale; rs-fMRI; spinal cord injury.
© The Author(s) 2024. Published by Mary Ann Liebert, Inc.
Figures


References
-
- Basso DM. Neuroanatomical substrates of functional recovery after experimental spinal cord injury: Implications of basic science research for human spinal cord injury. Phys Ther 2000;80(8):808–817. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
