SARS-CoV-2 immune responses in patients with multiple myeloma and lenalidomide maintenance therapy
- PMID: 39744626
- PMCID: PMC11688394
- DOI: 10.3389/fimmu.2024.1510942
SARS-CoV-2 immune responses in patients with multiple myeloma and lenalidomide maintenance therapy
Abstract
Introduction: Multiple myeloma (MM) is an uncontrolled plasma cell proliferation in the bone marrow, leading to immune dysregulation with impaired humoral immune responses. Conversely, cellular-based responses play a vital role in MM patients. However, the extent and duration of cellular-induced protection remain unclear to date. Here, immunomodulatory drugs (IMiDs) like Lenalidomide (Lena) become interesting, as they may have stimulatory effects on T-cell functioning.
Methods: In this study we investigated immune responses elicited by COVID-19 vaccine or infection comparing 43 healthy volunteers (avg. 35y, 72.1% female, 81.4% previously COVID-19 infected), with 41 MM patients under Lena maintenance therapy (avg. 63.8y, 51.2% female, 61% previously COVID-19 infected). Humoral responses to SARS-CoV-2 spike (S), spike-RBD, and nucleocapsid (N) were measured via ELISA in subjects' plasma. Freshly isolated PBMCs, incubated with SARS-CoV-2 peptides (N, S), activation induced marker (AIM) assays and flow cytometry, allowed us to assess cellular responses (CD8+ T, T(F)H: CD4+ T (follicular) helper).
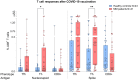
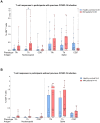
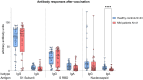
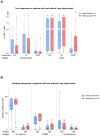
Results: Whereas healthy controls showed significant better humoral responses (N IgA p<0.001), T cell responses were robust in the MM group (higher S-act. TH, p<0.001). Stratified by COVID-19 status, the MM group showed higher N-act. TH (p=0.03). These results were unchanged comparing a Lena intake with Lena break around vaccination.
Discussion: Taken together, MM patients under Lena therapy exhibit weakened antibody production but present a robust T cell response following SARS-COV-2 infection or vaccination. Our results highlight the importance of vaccination in this subgroup and moreover, argue against a Lena intake break around the time of vaccination.
Keywords: cellular immune responses; immunomodulatory therapy; lenalidomide; multiple myeloma; vaccine response.
Copyright © 2024 Martac, Beer, Schenk, Ahmad, Maier, Demirel, Preuß, Klein, Stanger, Besemer, Hensen and Lengerke.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Figures


References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

