Tumor microenvironment in oral squamous cell carcinoma
- PMID: 39744628
- PMCID: PMC11688467
- DOI: 10.3389/fimmu.2024.1485174
Tumor microenvironment in oral squamous cell carcinoma
Abstract
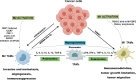
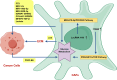
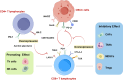
Oral squamous cell carcinoma (OSCC) is a highly aggressive and malignant tumor of oral cavity with a poor prognosis and high mortality due to the limitations of existing therapies. The significant role of tumor microenvironment (TME) in the initiation, development, and progression of OSCC has been widely recognized. Various cells in TME, including tumor-associated macrophages (TAMs), cancer-associated fibroblasts (CAFs), T lymphocytes, tumor-associated neutrophils (TANs), myeloid-derived suppressor cells (MDSCs) and dendritic cells (DCs), form a complicated and important cellular network to modulate OSCC proliferation, invasion, migration, and angiogenesis by secreting RNAs, proteins, cytokines, and metabolites. Understanding the interactions among cells in TME provides the foundation for advanced clinical diagnosis and therapies. This review summarizes the current literature that describes the role of various cellular components and other TME factors in the progression of OSCC, hoping to provide new ideas for the novel OSCC treatment strategies targeting the complicated cellular network and factors that mediate the interactive loops among cells in TME.
Keywords: cellular network; interaction; oral squamous cell carcinoma; tumor microenvironment; tumor-associated macrophages.
Copyright © 2024 Li, Dong and Li.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

