The landscape of chemokine and cytokine is associated with the distinct clinical status of leprosy patients and their respective household contacts
- PMID: 39744632
- PMCID: PMC11688302
- DOI: 10.3389/fimmu.2024.1476450
The landscape of chemokine and cytokine is associated with the distinct clinical status of leprosy patients and their respective household contacts
Abstract
Introduction: Leprosy, a chronic infectious disease, is closely linked to the host immune response. According to the WHO, leprosy patients (L) and household contacts (HHC) are classified into subgroups: paucibacillary (PB) and multibacillary (MB), witch reflect the degree of infection in patients and the level of exposure of their contacts. The main goal of this study was to: i) establish a comprehensive overview of soluble mediator signatures of PBMCs upon in vitro antigen-specific stimuli and ii) identify whether the chemokine (CH) and cytokine (CY) signatures were associated with distinct clinical manifestations in (L) and immune response profiles in (HHC).
Methods: Long-term PBMC cultures were carried out and supernatants collected for 12 CH and CY analisys by Cytometric Beads Array.
Results and discussion: The CH and CY analysis, using continuous variable modeling, demonstrated that PBMCs from both L and HHC exhibited high levels of TNF upon M. leprae-stimuli. While lower production of IFN-γ were observed for L, low levels of CXCL8 was found for HHC. Soluble mediator signatures, analyzed using categorical variables, revealed that while high levels of TNF were observed for L, high levels of IFN-γ appeared as a hallmark of HHC. Overall, these analyses demonstrated that CXCL8, IFN-γ, and TNF were key markers differentiating L from HHC and endemic control (EC), especially considering the categorical analysis of the soluble mediator signatures. Data further demonstrated that higher levels of IFN-γ and lower levels CXCL8 was features associated with HHC(MB), whereas high levels of TNF were observed in both L subgroups. Moreover, data from integrative networks, based on correlation amongst soluble mediators, revealed that in M. leprae-stimuli, the number of correlations was lower in HHC(MB) compared to HHC(PB), but higher in L(MB) compared to L(PB). It was noted that the number of correlations decreased in the following order: EC > L > HHC. Our findings contribute to additional immunological features associated with L and HHC, witch can be useful complementary diagnostic/prognostic tools for classification of L and HHC, providing insights to enrich the research agenda about the hypothesis that HHC should be closely monitored as they may present a subclinical infection.
Keywords: Mycobacterium leprae; chemokines; cytokines; household contacts; leprosy.
Copyright © 2024 Pereira de Oliveira, Marçal, Campos, dos Santos, Lima, Martins-Filho, Brito-de-Sousa, Abdala-Torres, Pinheiro, Sarno, Fairley and Fraga.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

 , n= 87), household contacts of leprosy patients (HHC –
, n= 87), household contacts of leprosy patients (HHC –  , n=91) and Leprosy patients (L, –
, n=91) and Leprosy patients (L, –  , n=79). HHC and L subjects were further classified into subgroups named paucibacillary and multibacillary, according to operational classification records and referred as to: [HHC(PB) –
, n=79). HHC and L subjects were further classified into subgroups named paucibacillary and multibacillary, according to operational classification records and referred as to: [HHC(PB) –  , n=20], [HHC(MB) –
, n=20], [HHC(MB) –  , n=78], [L(PB) –
, n=78], [L(PB) –  , n=23] and [L(MB) –
, n=23] and [L(MB) –  , n=56]. Heparinized whole blood samples (10mL) from were obtained from each participant and used to isolate PBMCs for experimental procedures to quantify soluble mediators by Cytometric Bead Array to quantify chemokines (CXCL8, CCL2, CXCL9, CCL5, CXCL10) and cytokines (IL-6, TNF, IFN-γ, IL-17, IL-4, IL-10 and IL-2) in cell culture supernatants obtained in the absence on exogenous stimuli and in the presence of M. leprae stimuli. Distinct approaches were employed for data mining and statistics, including: conventional statistics, soluble mediator signatures and networks. Systems immunology tools were employed to assemble integrative network, color maps and fold change constructs.
, n=56]. Heparinized whole blood samples (10mL) from were obtained from each participant and used to isolate PBMCs for experimental procedures to quantify soluble mediators by Cytometric Bead Array to quantify chemokines (CXCL8, CCL2, CXCL9, CCL5, CXCL10) and cytokines (IL-6, TNF, IFN-γ, IL-17, IL-4, IL-10 and IL-2) in cell culture supernatants obtained in the absence on exogenous stimuli and in the presence of M. leprae stimuli. Distinct approaches were employed for data mining and statistics, including: conventional statistics, soluble mediator signatures and networks. Systems immunology tools were employed to assemble integrative network, color maps and fold change constructs.
 , n=79), household contacts (HHC =
, n=79), household contacts (HHC =  , n=91) and endemic controls (EC =
, n=91) and endemic controls (EC =  , n=87). Data were obtained (A) in the absence of exogenous stimuli (Unstimulated Culture = open bars) and (B) in the presence of M. leprae antigen stimuli (M. leprae-stimulated Culture = filled bars). Cytometric Beads Array (CBA) performed quantitative analysis of chemokines and cytokines according to manufacturer instructions. The results are expressed in pg/mL and presented as a scatter distribution of individual values over bars showing mean values. Comparative analysis amongst groups was carried out by Kruskal-Wallis test followed by Dunn’s post-test for multiple comparisons amongst EC vs HHC vs L subgroups. In all cases, significant differences were considered at p<0.05. Kruskal-Wallis p values are provided for each parameter and the significant differences amongst groups identified by Dunn’s post-test are indicated by connecting lines and * (p<0.05), ** (p<0.01), *** (p<0.001) and **** (p<0.0001).
, n=87). Data were obtained (A) in the absence of exogenous stimuli (Unstimulated Culture = open bars) and (B) in the presence of M. leprae antigen stimuli (M. leprae-stimulated Culture = filled bars). Cytometric Beads Array (CBA) performed quantitative analysis of chemokines and cytokines according to manufacturer instructions. The results are expressed in pg/mL and presented as a scatter distribution of individual values over bars showing mean values. Comparative analysis amongst groups was carried out by Kruskal-Wallis test followed by Dunn’s post-test for multiple comparisons amongst EC vs HHC vs L subgroups. In all cases, significant differences were considered at p<0.05. Kruskal-Wallis p values are provided for each parameter and the significant differences amongst groups identified by Dunn’s post-test are indicated by connecting lines and * (p<0.05), ** (p<0.01), *** (p<0.001) and **** (p<0.0001).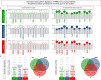
 ,
,  , n=79), household contacts (HHC =
, n=79), household contacts (HHC =  ,
,  , n=91) and endemic controls (EC =
, n=91) and endemic controls (EC =  ,
,  , n=87). Data were obtained in the absence of exogenous stimuli (Unstimulated Culture = open circles) and the presence of M. leprae antigen stimuli (M. leprae-stimulated Culture = filled circles). Cytometric Beads Array (CBA) performed quantitative analysis of chemokines and cytokines according to manufacturer instructions. The results are reported as chemokine and cytokine signatures as described in the methods. Continuous variables expressed in pg/mL were converted into categorical data using the intrinsic median values of unstimulated culture or M. leprae stimulated culture as the cut-off to identify volunteers with low and high soluble mediators as described in methods. (A) The proportion of subjects (%) with chemokine and cytokine levels above the intrinsic cut-off was calculated for each study group, and data are presented as lollipop charts. The chemokines and cytokines with the proportion of subjects above the 50th percentile were underscored and considered as increased levels were underscored by color font format. Comparative analysis between HHC vs EC and L vs EC was carried out by Chi-square and significant differences indicated by * (p<0.05), ** (p<0.01) and *** (p<0.001). (B) The chemokines and cytokines signatures were further assembled as ascendant signatures, and Venn diagrams were constructed to identify common and selective soluble mediators amongst groups.
, n=87). Data were obtained in the absence of exogenous stimuli (Unstimulated Culture = open circles) and the presence of M. leprae antigen stimuli (M. leprae-stimulated Culture = filled circles). Cytometric Beads Array (CBA) performed quantitative analysis of chemokines and cytokines according to manufacturer instructions. The results are reported as chemokine and cytokine signatures as described in the methods. Continuous variables expressed in pg/mL were converted into categorical data using the intrinsic median values of unstimulated culture or M. leprae stimulated culture as the cut-off to identify volunteers with low and high soluble mediators as described in methods. (A) The proportion of subjects (%) with chemokine and cytokine levels above the intrinsic cut-off was calculated for each study group, and data are presented as lollipop charts. The chemokines and cytokines with the proportion of subjects above the 50th percentile were underscored and considered as increased levels were underscored by color font format. Comparative analysis between HHC vs EC and L vs EC was carried out by Chi-square and significant differences indicated by * (p<0.05), ** (p<0.01) and *** (p<0.001). (B) The chemokines and cytokines signatures were further assembled as ascendant signatures, and Venn diagrams were constructed to identify common and selective soluble mediators amongst groups.
 ;
;  , n=79), household contacts (HHC =
, n=79), household contacts (HHC =  ;
;  , n=91) and endemic controls (EC =
, n=91) and endemic controls (EC =  ,
,  , n=87). Data were obtained in the absence of exogenous stimuli (Unstimulated Culture = open circles) and the presence of M. leprae antigen stimuli (M. leprae-stimulated Culture = filled circles). Cytometric Beads Array (CBA) performed quantitative analysis of chemokines and cytokines according to manufacturer instructions. Integrative networks were built based on correlation analysis (Spearman rank tests) between pairs of soluble mediators, and significant correlations (p<0.05) were employed to construct networks using the open-source Cytoscape software as described in the methods. (A) The networks were assembled using cluster layouts with nodes representing each chemokine and cytokine (numbered as provided in the figure) and connecting lines identifying positive (“r” scores >0, continuous line) or negative (“r” scores <0, dashed line) correlations. The node sizes are proportional to the correlations between pairs of soluble mediators. Comparative analysis amongst groups was carried out considering the total number of correlations. (B) Colormaps analysis of integrative networks illustrates the comparison amongst groups using a color key based on the percentile distribution (10th/50th/90th) of correlation numbers calculated for soluble mediator, chemokine, and cytokine clusters or the total number of correlations.
, n=87). Data were obtained in the absence of exogenous stimuli (Unstimulated Culture = open circles) and the presence of M. leprae antigen stimuli (M. leprae-stimulated Culture = filled circles). Cytometric Beads Array (CBA) performed quantitative analysis of chemokines and cytokines according to manufacturer instructions. Integrative networks were built based on correlation analysis (Spearman rank tests) between pairs of soluble mediators, and significant correlations (p<0.05) were employed to construct networks using the open-source Cytoscape software as described in the methods. (A) The networks were assembled using cluster layouts with nodes representing each chemokine and cytokine (numbered as provided in the figure) and connecting lines identifying positive (“r” scores >0, continuous line) or negative (“r” scores <0, dashed line) correlations. The node sizes are proportional to the correlations between pairs of soluble mediators. Comparative analysis amongst groups was carried out considering the total number of correlations. (B) Colormaps analysis of integrative networks illustrates the comparison amongst groups using a color key based on the percentile distribution (10th/50th/90th) of correlation numbers calculated for soluble mediator, chemokine, and cytokine clusters or the total number of correlations.
 ), increase (
), increase ( ), or non-significant changes (
), or non-significant changes ( ) are highlighted on each chart distribution.
) are highlighted on each chart distribution.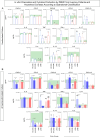
 ;
;  , n=23 and L(MB) =
, n=23 and L(MB) =  ;
;  , n= 56] and household contacts [HHC(PB) =
, n= 56] and household contacts [HHC(PB) =  ;
;  , n=20 and HHC(MB) =
, n=20 and HHC(MB) =  ;
;  , n= 68] subgroups for comparisons with endemic controls (EC = green reference 95%CI range, n=87). Data were obtained (A) in the absence of exogenous stimuli (Unstimulated Culture = open bars) and (B) in the presence of M. leprae antigen stimuli (M. leprae-stimulated Culture = filled bars). Cytometric Beads Array (CBA) performed quantitative analysis of chemokines and cytokines according to manufacturer instructions. The results are expressed in pg/mL and presented in bar charts showing mean values and standard error. Comparative analysis amongst groups was carried out by Kruskal-Wallis test followed by Dunn’s post-test for multiple comparisons amongst EC vs HHC(PB) vs HHC(MB) vs L(PB) vs L(PB) subgroups. In all cases, significant differences were considered at p<0.05. Kruskal-Wallis p values are provided for each parameter and the significant differences amongst groups identified by Dunn’s post-test are indicated by § for comparisons with EC, # for intergroup comparisons and connecting lines with * for MB vs PB intragroup comparisons. In all case, the number of symbols (1, 2, 3 and 4) indicated the power of p values (p<0.05, p<0.01, p<0.001 and p<0.0001, respectively).
, n= 68] subgroups for comparisons with endemic controls (EC = green reference 95%CI range, n=87). Data were obtained (A) in the absence of exogenous stimuli (Unstimulated Culture = open bars) and (B) in the presence of M. leprae antigen stimuli (M. leprae-stimulated Culture = filled bars). Cytometric Beads Array (CBA) performed quantitative analysis of chemokines and cytokines according to manufacturer instructions. The results are expressed in pg/mL and presented in bar charts showing mean values and standard error. Comparative analysis amongst groups was carried out by Kruskal-Wallis test followed by Dunn’s post-test for multiple comparisons amongst EC vs HHC(PB) vs HHC(MB) vs L(PB) vs L(PB) subgroups. In all cases, significant differences were considered at p<0.05. Kruskal-Wallis p values are provided for each parameter and the significant differences amongst groups identified by Dunn’s post-test are indicated by § for comparisons with EC, # for intergroup comparisons and connecting lines with * for MB vs PB intragroup comparisons. In all case, the number of symbols (1, 2, 3 and 4) indicated the power of p values (p<0.05, p<0.01, p<0.001 and p<0.0001, respectively).
 ;
;  , n=23 and L(MB) =
, n=23 and L(MB) =  ;
;  , n= 56] and household contacts [HHC(PB) =
, n= 56] and household contacts [HHC(PB) =  ;
;  , n=20 and HHC(MB) =
, n=20 and HHC(MB) =  ;
;  , n= 68] subgroups. Data were obtained in the absence of exogenous stimuli (Unstimulated Culture = open circles) and the presence of M. leprae antigen stimuli (M. leprae-stimulated Culture = filled circles). Cytometric Beads Array (CBA) performed quantitative analysis of chemokines and cytokines according to manufacturer instructions. The results are reported as chemokine and cytokine signatures as described in the methods. Continuous variables expressed in pg/mL were converted into categorical data using the intrinsic median values of unstimulated culture or M. leprae stimulated culture as the cut-off to identify volunteers with low and high soluble mediators as described in methods. (A) The proportion of subjects (%) with chemokine and cytokine levels above the intrinsic cut-off was calculated for each study subgroup, and data are presented as line charts. The chemokines and cytokines with the proportion of subjects above the 50th percentile were underscored and considered as increased levels were underscored by color font format. Intragroup comparisons between MB vs PB was carried out by Chi-square and significant differences indicated by * (p<0.05), ** (p<0.01), *** (p<0.001) and **** (p<0.0001). (B) The chemokines and cytokines signatures were assembled as ascendant signatures and subgroup-selective soluble mediators underscored by #. Colormaps illustrate the comparison amongst subgroups using a color key based on the proportion of subjects above the 50th percentile (0/50/100).
, n= 68] subgroups. Data were obtained in the absence of exogenous stimuli (Unstimulated Culture = open circles) and the presence of M. leprae antigen stimuli (M. leprae-stimulated Culture = filled circles). Cytometric Beads Array (CBA) performed quantitative analysis of chemokines and cytokines according to manufacturer instructions. The results are reported as chemokine and cytokine signatures as described in the methods. Continuous variables expressed in pg/mL were converted into categorical data using the intrinsic median values of unstimulated culture or M. leprae stimulated culture as the cut-off to identify volunteers with low and high soluble mediators as described in methods. (A) The proportion of subjects (%) with chemokine and cytokine levels above the intrinsic cut-off was calculated for each study subgroup, and data are presented as line charts. The chemokines and cytokines with the proportion of subjects above the 50th percentile were underscored and considered as increased levels were underscored by color font format. Intragroup comparisons between MB vs PB was carried out by Chi-square and significant differences indicated by * (p<0.05), ** (p<0.01), *** (p<0.001) and **** (p<0.0001). (B) The chemokines and cytokines signatures were assembled as ascendant signatures and subgroup-selective soluble mediators underscored by #. Colormaps illustrate the comparison amongst subgroups using a color key based on the proportion of subjects above the 50th percentile (0/50/100).
 ;
;  , n=23 and L(MB) =
, n=23 and L(MB) =  ;
;  , n= 56] and household contacts [HHC(PB) =
, n= 56] and household contacts [HHC(PB) =  ;
;  , n=20 and HHC(MB) =
, n=20 and HHC(MB) =  ;
;  , n= 68] subgroups. Data were obtained in the absence of exogenous stimuli (Unstimulated Culture = open circles) and the presence of M. leprae antigen stimuli (M. leprae-stimulated Culture = filled circles). Cytometric Beads Array (CBA) performed quantitative analysis of chemokines and cytokines according to manufacturer instructions. Integrative networks were built based on correlation analysis (Spearman rank tests) between pairs of soluble mediators, and significant correlations (p<0.05) were employed to construct networks using the open-source Cytoscape software as described in the methods. (A) The networks were assembled using cluster layouts with nodes representing each chemokine and cytokine (numbered as provided in the figure) and connecting lines identifying positive (“r” scores >0, continuous line) or negative (“r” scores <0, dashed line) correlations. The node sizes are proportional to the correlations between pairs of soluble mediators. Comparative analysis amongst subgroups was carried out considering the total number of correlations. (B) Colormaps analysis of integrative networks illustrates the comparison amongst subgroups using a color key based on the percentile distribution (10th/50th/90th) of correlation numbers calculated for soluble mediator, chemokine, and cytokine clusters or the total number of correlations.
, n= 68] subgroups. Data were obtained in the absence of exogenous stimuli (Unstimulated Culture = open circles) and the presence of M. leprae antigen stimuli (M. leprae-stimulated Culture = filled circles). Cytometric Beads Array (CBA) performed quantitative analysis of chemokines and cytokines according to manufacturer instructions. Integrative networks were built based on correlation analysis (Spearman rank tests) between pairs of soluble mediators, and significant correlations (p<0.05) were employed to construct networks using the open-source Cytoscape software as described in the methods. (A) The networks were assembled using cluster layouts with nodes representing each chemokine and cytokine (numbered as provided in the figure) and connecting lines identifying positive (“r” scores >0, continuous line) or negative (“r” scores <0, dashed line) correlations. The node sizes are proportional to the correlations between pairs of soluble mediators. Comparative analysis amongst subgroups was carried out considering the total number of correlations. (B) Colormaps analysis of integrative networks illustrates the comparison amongst subgroups using a color key based on the percentile distribution (10th/50th/90th) of correlation numbers calculated for soluble mediator, chemokine, and cytokine clusters or the total number of correlations.
 ), increase (
), increase ( ), or non-significant changes (
), or non-significant changes ( ) are highlighted on each chart distribution.
) are highlighted on each chart distribution.References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

