Efficacy analysis of rituximab in treating patients with primary membranous nephropathy dependent on calcineurin inhibitors
- PMID: 39744641
- PMCID: PMC11688482
- DOI: 10.3389/fimmu.2024.1504646
Efficacy analysis of rituximab in treating patients with primary membranous nephropathy dependent on calcineurin inhibitors
Abstract
Background: This study evaluated the efficacy of rituximab (RTX) in primary membranous nephropathy (PMN) patients with incomplete remission and drug dependence after long-term use of calmodulin inhibitors (CNIs). It aims for complete clinical and immunological remission, and cessation of CNI dependence.
Methods: Thirty-six patients were enrolled in the study with two groups: drug-dependent and partial remission or immune non-remission group. Both groups underwent RTX therapy with gradual CNI tapering to end CNI dependency and induce complete remission. The primary outcome was overcoming CNI dependency and achieving complete remission after 12 months of RTX therapy. Secondary outcomes included immunological remission and recurrence rates.
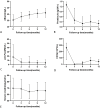
Results: The drug-dependent group (20 patients) achieved significant proteinuria reduction compared to the partial remission or immune non-remission group (16 patients) (P=0.016). After 12 months of RTX treatment, all drug-dependent patients overcame CNI dependency (average withdrawal period: 5.3 ± 3.7 months), with complete remission rates increased from 10% to 70.0% and complete immunological remission rates rose from 35.0% to 90.0%. In the partial remission or immune non-remission group, 14 patients discontinued CNI (average period: 4.6 ± 4.5 months), with complete remission rates increasing from 5.0% to 68.8% and complete immunological remission rates from 6.3% to 68.8%. During follow-up, serum albumin increased, and anti-PLA2R antibodies, 24-hour proteinuria, and CD19+ cell numbers reduced, while creatinine remained stable. Three patients relapsed, four encountered adverse events, and no malignancies or other fatal adverse events were reported.
Conclusions: RTX effectively achieves complete clinical and immunological remission in PMN patients dependent on or partially responsive to long-term CNI therapy, reducing recurrence and minimizing prolonged immunosuppressive therapy risks.
Keywords: clinical remission rate; immunologic remission rate; primary membranous nephropathy; rituximab; withdrawal drug rate.
Copyright © 2024 Li, Zhao, Zhang, Huang, Wang, Sun, Wang and Chen.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
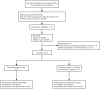
Figures

References
-
- Allinovi M, Lugli G, Rossi F, Palterer B, Almerigogna F, Caroti L, et al. Accuracy of serum Pla2r antibody detected by indirect immunofluorescence in diagnosing biopsy-proven primary membranous nephropathy: A single-center experience and a systematic review of the literature. J Nephrol. (2023) 36:281–3. doi: 10.1007/s40620-022-01528-1 - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

