Unraveling the SARS-CoV-2 spike protein long-term effect on neuro-PASC
- PMID: 39744674
- PMCID: PMC11688492
- DOI: 10.3389/fncel.2024.1481963
Unraveling the SARS-CoV-2 spike protein long-term effect on neuro-PASC
Abstract
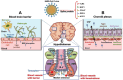
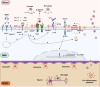
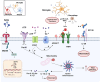
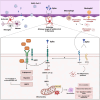
The persistence or emergence of long-term symptoms following resolution of primary SARS-CoV-2 infection is referred to as long COVID or post-acute sequelae of COVID-19 (PASC). PASC predominantly affects the cardiovascular, neurological, respiratory, gastrointestinal, reproductive, and immune systems. Among these, the central nervous system (CNS) is significantly impacted, leading to a spectrum of symptoms, including fatigue, headaches, brain fog, cognitive impairment, anosmia, hypogeusia, neuropsychiatric symptoms, and peripheral neuropathy (neuro-PASC). However, the risk factors and pathogenic mechanisms responsible for neuro-PASC remain unclear. This review hypothesis discusses the leading hypotheses regarding the pathophysiological mechanisms involved in long COVID/PASC, focusing on neuro-PASC. We propose vascular dysfunction mediated by activation of astrocytes and pericytes followed by blood-brain barrier (BBB) disruption as underlying pathophysiological mechanisms of neurological manifestations. Additionally, we provide insights into the role of spike protein at the blood-brain interface. Finally, we explore the potential pathogenic mechanisms initiated by the interaction between the spike protein and cellular receptors at the brain endothelial and tissue levels.
Keywords: SARS-CoV-2 receptors; SARS-CoV-2 spike protein; blood–brain barrier; neuro-PASC; pathophysiology.
Copyright © 2024 Menezes, Palmeira, Oliveira, Argañaraz, Soares, Nóbrega, Ribeiro and Argañaraz.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- Agrawal S., Farfel J. M., Arfanakis K., Al-Harthi L., Shull T., Teppen T. L., et al. (2022). Brain autopsies of critically ill COVID-19 patients demonstrate heterogeneous profile of acute vascular injury, inflammation and age-linked chronic brain diseases. Acta Neuropathol. Commun. 10:186. doi: 10.1186/s40478-022-01493-7, PMID: - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

