Inhibition of nitric oxide synthase transforms carotid occlusion-mediated benign oligemia into de novo large cerebral infarction
- PMID: 39744690
- PMCID: PMC11671383
- DOI: 10.7150/thno.104132
Inhibition of nitric oxide synthase transforms carotid occlusion-mediated benign oligemia into de novo large cerebral infarction
Abstract
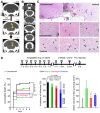
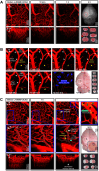
Rationale: It remains unclear why unilateral proximal carotid artery occlusion (UCAO) causes benign oligemia in mice, yet leads to various outcomes (asymptomatic-to-death) in humans. We hypothesized that inhibition of nitric oxide synthase (NOS) both transforms UCAO-mediated oligemia into full infarction and expands pre-existing infarction. Methods: Using 900 mice, we i) investigated stroke-related effects of UCAO with/without intraperitoneal administration of the NOS inhibitor (NOSi) Nω-nitro-L-arginine methyl ester (L-NAME, 400 mg/kg); ii) examined the rescue effect of the NO-donor, molsidomine (200 mg/kg at 30 minutes); and iii) tested the impact of antiplatelet medications. To corroborate preclinical findings, we conducted clinical studies. Results: UCAO alone induced infarction rarely (~2%) or occasionally (~14%) in C57BL/6 and BALB/c mice, respectively. However, L-NAME+UCAO induced large-arterial infarction in ~75% of C57BL/6 and BALB/c mice. Six-hour laser-speckle imaging detected spreading ischemia in ~40% of C57BL/6 and BALB/c mice with infarction (vs. none without) by 24-hours. In agreement with vasoconstriction/microthrombus formation shown by intravital-microscopy, molsidomine and the endothelial-NOS-activating antiplatelet cilostazol attenuated/prevented progression to infarction. Moreover, UCAO without L-NAME caused infarction in ~22% C57BL/6 and ~31% ApoE knock-out mice with hyperglycemia/hyperlipidemia, which associated with ~60% greater levels of symmetric dimethylarginine (SDMA, an endogenous NOSi). Further, increased levels of glucose and cholesterol associated with significantly larger infarct volumes in 438 UCAO-stroke patients. Lastly, Mendelian randomization identified a causative role of NOS inhibition (elevated SDMA concentration) in ischemic stroke risk (OR = 1.24; 95% CI, 1.11-1.38; P = 7.69×10-5). Conclusion: NOS activity determines the fate of hypoperfused brain following acute UCAO, where SDMA could be a potential risk predictor.
Keywords: carotid artery occlusion; cerebral infarction; nitric oxide synthase; oligemia; stroke.
© The author(s).
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures





References
-
- Flaherty ML, Flemming KD, McClelland R, Jorgensen NW, Brown RD Jr. Population-based study of symptomatic internal carotid artery occlusion: incidence and long-term follow-up. Stroke. 2004;35:e349–52. - PubMed
-
- Kluytmans M, van der Grond J, van Everdingen KJ, Klijn CJ, Kappelle LJ, Viergever MA. Cerebral hemodynamics in relation to patterns of collateral flow. Stroke. 1999;30:1432–9. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

