Inhibition of Bruton's tyrosine kinase restricts neuroinflammation following intracerebral hemorrhage
- PMID: 39744694
- PMCID: PMC11671375
- DOI: 10.7150/thno.101024
Inhibition of Bruton's tyrosine kinase restricts neuroinflammation following intracerebral hemorrhage
Abstract
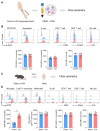
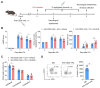
Background: Intracerebral hemorrhage (ICH) is a devastating form of stroke with a lack of effective treatments. Following disease onset, ICH activates microglia and recruits peripheral leukocytes into the perihematomal region to amplify neural injury. Bruton's tyrosine kinase (BTK) controls the proliferation and survival of various myeloid cells and lymphocytes. However, the role of BTK in neuroinflammation and ICH injury remains poorly understood. Methods: Peripheral blood samples were collected from ICH patients and healthy controls to measure BTK expression profile in immune cell subsets. C57BL/6 mice were used to measure BTK expression and the activity of immune cell subsets following ICH induction. Neurological tests, brain water content, Evans blue leakage, MRI were used to assess the therapeutic effects of ibrutinib on ICH injury. Flow cytometry was used to investigate immune cell infiltration and microglial activity. Microglia were depleted using a CSF1R inhibitor PLX5622. Gr-1+ myeloid cells and B cells were depleted using monoclonal antibodies. Microglia-like BV2 cells were cultured to test the effects of BTK inhibition on these cells. Results: In humans and mice, we found that BTK was remarkably upregulated in myeloid cells after ICH. Inhibition of BTK using ibrutinib led to reduced neurological deficits, perihematomal edema, brain water content and blood-brain barrier disruption. BTK inhibition suppressed the inflammatory activity of microglia and brain infiltration of leukocytes. In contrast, BTK inhibition did not alter the counts of peripheral immune cells other than B cells. Further, the depletion of microglia or Gr-1+ myeloid cells ablated the protective effects of BTK inhibition against ICH injury. Notably, the depletion of B cells did not alter the protective effects of BTK inhibition against ICH injury. This suggests that the benefit of BTK inhibition in ICH mainly involves its impact on microglia and Gr-1+ myeloid cells. Conclusion: Our findings demonstrate that BTK inhibition attenuates neuroinflammation and ICH injury, which warrants further investigation as a potential therapy for ICH.
Keywords: Bruton's tyrosine kinase; Ibrutinib.; Intracerebral hemorrhage; Microglia; Neuroinflammation.
© The author(s).
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures





References
-
- Greenberg SM, Ziai WC, Cordonnier C, Dowlatshahi D, Francis B, Goldstein JN. et al. 2022 Guideline for the Management of Patients With Spontaneous Intracerebral Hemorrhage: A Guideline From the American Heart Association/American Stroke Association. Stroke. 2022;53:e282–e361. - PubMed
-
- Hanley DF, Thompson RE, Rosenblum M, Yenokyan G, Lane K, McBee N. et al. Efficacy and safety of minimally invasive surgery with thrombolysis in intracerebral haemorrhage evacuation (MISTIE III): a randomised, controlled, open-label, blinded endpoint phase 3 trial. Lancet. 2019;393:1021–32. - PMC - PubMed
-
- Wang X, Arima H, Yang J, Zhang S, Wu G, Woodward M. et al. Mannitol and Outcome in Intracerebral Hemorrhage. Stroke. 2015;46:2762–7. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

