LIM kinases in cardiovascular health and disease
- PMID: 39744707
- PMCID: PMC11688343
- DOI: 10.3389/fphys.2024.1506356
LIM kinases in cardiovascular health and disease
Abstract
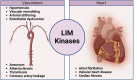
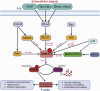
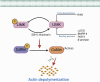
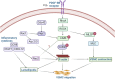
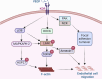
The Lim Kinase (LIMK) family of serine/threonine kinases is comprised of LIMK1 and LIMK2, which are central regulators of cytoskeletal dynamics via their well-characterized roles in promoting actin polymerization and destabilizing the cellular microtubular network. The LIMKs have been demonstrated to modulate several fundamental physiological processes, including cell cycle progression, cell motility and migration, and cell differentiation. These processes play important roles in maintaining cardiovascular health. However, LIMK activity in healthy and pathological states of the cardiovascular system is poorly characterized. This review highlights the cellular and molecular mechanisms involved in LIMK activation and inactivation, examining its roles in the pathophysiology of vascular and cardiac diseases such as hypertension, aneurysm, atrial fibrillation, and valvular heart disease. It addresses the LIMKs' involvement in processes that support cardiovascular health, including vasculogenesis, angiogenesis, and endothelial mechanotransduction. The review also features how LIMK activity participates in endothelial cell, vascular smooth muscle cell, and cardiomyocyte physiology and its implications in pathological states. A few recent preclinical studies demonstrate the therapeutic potential of LIMK inhibition. We conclude by proposing that future research should focus on the potential clinical relevance of LIMK inhibitors as therapeutic agents to reduce the burden of cardiovascular disease and improve patient outcomes.
Keywords: arterial stiffening; atherosclerosis; atrial fibrillation; cardiovascular disease; hypertension; vascular remodeling.
Copyright © 2024 Lateef, Foote, Power, Manrique-Acevedo, Padilla and Martinez-Lemus.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

