Bacterial attachment and junctional transport function in induced apical-out polarized and differentiated canine intestinal organoids
- PMID: 39744718
- PMCID: PMC11688377
- DOI: 10.3389/fvets.2024.1483421
Bacterial attachment and junctional transport function in induced apical-out polarized and differentiated canine intestinal organoids
Abstract
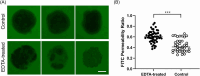
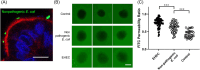
Dogs are increasingly recognized as valuable large animal models for understanding human intestinal diseases, as they naturally develop conditions similar to those in humans, such as Enterohemorrhagic E. coli, Clostridium difficile infection, inflammatory bowel disease, and ulcerative colitis. Given the similarity in gut flora between dogs and humans, canine in vitro intestinal models are ideal for translational research. However, conventional extracellular matrix-embedded organoids present challenges in accessing the lumen, which is critical for gut function. This study aimed to investigate the feasibility of inducing polarity reversal and differentiation in canine apical-out colonic organoids (colonoids), evaluate their barrier integrity, and visualize host-pathogen interactions. Our results demonstrated successful polarity reversal and differentiation induction while maintaining barrier integrity. Polarity reversal allowed for enhanced observation of host-pathogen interactions, facilitating visual assessments and membrane integrity evaluations using both pathogenic and nonpathogenic E. coli. This process led to the downregulation of stem cell marker LGR5 and upregulation of intestinal epithelial cell marker ALPI, indicating differentiation. Further differentiation was observed with the use of a differentiation culture medium, resulting in significant upregulation of ALPI and goblet cell marker MUC2. The findings suggest that apical-out canine colonoids can serve as physiologic and valuable models for studying the pathogenic mechanisms and clinical significance of intestinal diseases in dogs. This model has the potential to advance both canine and human gastrointestinal research, enhancing our understanding of gastrointestinal physiology and pathology and aiding in the development of novel therapeutics.
Keywords: apical-out; canine; differentiation; host-pathogen interaction; intestine; organoid; polarity reversal.
Copyright © 2024 Yoshida, Nakazawa, Kawasaki and Ambrosini.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
-
- Sindern N, Suchodolski JS, Leutenegger CM, Gohari IM, Prescott JF, Proksch A, et al. . Prevalence of Clostridium perfringens netE and netF toxin genes in the feces of dogs with acute hemorrhagic diarrhea syndrome. J Vet Intern Med. (2019) 33:100–5. doi: 10.1111/jvim.15361, PMID: - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

