Cell migration signaling through the EGFR-VAV2-Rac1 pathway is sustained in endosomes
- PMID: 39744818
- PMCID: PMC11828472
- DOI: 10.1242/jcs.263541
Cell migration signaling through the EGFR-VAV2-Rac1 pathway is sustained in endosomes
Abstract
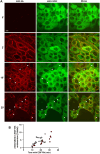
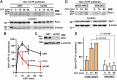
Ligand binding to EGFR activates Rho family GTPases, triggering actin cytoskeleton reorganization, cell migration and invasion. Activated EGFR is also rapidly endocytosed but the role of EGFR endocytosis in cell motility is poorly understood. Hence, we used live-cell microscopy imaging to demonstrate that endogenous fluorescently labeled VAV2, a guanine nucleotide exchange factor for Rho GTPases, is co-endocytosed with EGFR in genome-edited human oral squamous cell carcinoma (HSC3) cells, an in vitro model for head-and-neck cancer where VAV2 is known to promote metastasis and is associated with poor prognosis. Chemotactic migration of HSC3 cells toward an EGF gradient is found to require both VAV2 and clathrin-mediated endocytosis. Moreover, sustained activation of Rac1, a Rho family GTPase promoting cell migration and a major substrate of VAV2, also depends on clathrin. Endogenous fluorescently labeled Rac1 localizes to EGFR-containing endosomes. Altogether, our findings suggest that signaling through the EGFR-VAV2-Rac1 pathway persists in endosomes and that this endosomal signaling is required for EGFR-driven cell migration.
Keywords: Cell motility; EGFR; Endocytosis; Rac1; VAV2.
© 2025. Published by The Company of Biologists.
Conflict of interest statement
Competing interests The authors declare no competing or financial interests.
Figures





References
-
- Duan, L., Raja, S. M., Chen, G., Virmani, S., Williams, S. H., Clubb, R. J., Mukhopadhyay, C., Rainey, M. A., Ying, G., Dimri, M.et al. (2011). Negative regulation of EGFR-Vav2 signaling axis by Cbl ubiquitin ligase controls EGF receptor-mediated epithelial cell adherens junction dynamics and cell migration. J. Biol. Chem. 286, 620-633. 10.1074/jbc.M110.188086 - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

