Tandem Versus Single Autologous Stem Cell Transplantation for High-Risk Multiple Myeloma in the Era of Novel Agents: A Real-World Study of China
- PMID: 39744915
- PMCID: PMC11694139
- DOI: 10.1002/cam4.70573
Tandem Versus Single Autologous Stem Cell Transplantation for High-Risk Multiple Myeloma in the Era of Novel Agents: A Real-World Study of China
Abstract
Background: This study compares the efficacy and safety of single autologous stem cell transplantation (ASCT) versus tandem ASCT for multiple myeloma (MM) patients in the era of novel agents.
Methods: A total of 112 high-risk MM patients were included (single ASCT, (n = 57) or tandem ASCT(n = 55) in this retrospective multicenter study. Responses and outcomes were evaluated.
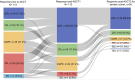
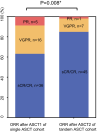
Results: At 100 days after ASCT1 and ASCT2, 36 (63.2%) versus 45 (81.8%) patients achieved sCR/CR, 16 (28.1%) versus 7 (12.7%) patients achieved VGPR, and 5 (8.8%) versus 1 (1.8%) patient achieved PR, respectively, in the single and tandem ASCT cohorts. The 3-year cumulative incidence of non-relapse mortality and disease progression was 0% versus 7.3% (p = 0.083), and 45.8% versus 25.8% (p = 0.039), respectively, for the single and tandem ASCT cohort. The tandem ASCT cohort showed a trend of better 3-year probability of PFS (58.1% vs. 64.7%, p = 0.064) compared with the single ASCT cohort. In multivariate analysis, ultra high-risk and achieving<VGPR response after ASCT1 were associated with an inferior PFS. Ultra high-risk was also associated with an inferior OS.
Conclusions: Tandem ASCT demonstrated improved outcomes compared to single ASCT in high-risk MM patients receiving triplet or quadruplet induction and maintenance therapy. However, patients with ultra high-risk cytogenetics may require innovative therapeutic approaches, as tendem ASCT does not overcome their adverse prognosis.
Keywords: autologous stem cell transplant; multiple myeloma; tandem.
© 2025 The Author(s). Cancer Medicine published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


Similar articles
-
Autologous stem cell transplantation in first remission is associated with better progression-free survival in multiple myeloma.Ann Hematol. 2018 Oct;97(10):1869-1877. doi: 10.1007/s00277-018-3370-1. Epub 2018 May 21. Ann Hematol. 2018. PMID: 29781040
-
Impact of autologous stem cell transplantation (ASCT) on progression free survival (PFS) in newly diagnosed multiple myeloma patients (NDMM) with high risk cytogenetic abnormalities.Bratisl Lek Listy. 2024;125(1):9-11. doi: 10.4149/BLL_2024_002. Bratisl Lek Listy. 2024. PMID: 38041839
-
Consolidation With Second High Dose Therapy and Autologous Stem Cell Transplantation Is Associated With Improved Overall Survival in Patients With Multiple Myeloma in First Relapse.Clin Lymphoma Myeloma Leuk. 2025 May;25(5):357-364.e5. doi: 10.1016/j.clml.2024.12.010. Epub 2024 Dec 24. Clin Lymphoma Myeloma Leuk. 2025. PMID: 39824724
-
Deepening Responses after Upfront Autologous Stem Cell Transplantation in Patients with Newly Diagnosed Multiple Myeloma in the Era of Novel Agent Induction Therapy.Transplant Cell Ther. 2022 Nov;28(11):760.e1-760.e5. doi: 10.1016/j.jtct.2022.07.030. Epub 2022 Aug 5. Transplant Cell Ther. 2022. PMID: 35940527 Free PMC article.
-
The Progress of Autologous Hematopoietic Stem Cell Transplantation in the Treatment of Multiple Myeloma (Review).Technol Cancer Res Treat. 2025 Jan-Dec;24:15330338251321349. doi: 10.1177/15330338251321349. Epub 2025 Mar 25. Technol Cancer Res Treat. 2025. PMID: 40129396 Free PMC article. Review.
Cited by
-
Clinical benefit loss in myeloma patients declining autologous stem cell transplantation: a real-world study.Discov Oncol. 2025 Apr 16;16(1):534. doi: 10.1007/s12672-025-02356-y. Discov Oncol. 2025. PMID: 40238028 Free PMC article.
-
Comparison of tandem and single autologous stem cell transplantation in multiple myeloma: a retrospective propensity score-matching study.Blood Sci. 2025 May 9;7(2):e00235. doi: 10.1097/BS9.0000000000000235. eCollection 2025 Jun. Blood Sci. 2025. PMID: 40356608 Free PMC article. No abstract available.
References
-
- Gay F., Musto P., Rota‐Scalabrini D., et al., “Carfilzomib With Cyclophosphamide and Dexamethasone or Lenalidomide and Dexamethasone Plus Autologous Transplantation or Carfilzomib Plus Lenalidomide and Dexamethasone, Followed by Maintenance With Carfilzomib Plus Lenalidomide or Lenalidomide Alone for Patients With Newly Diagnosed Multiple Myeloma (FORTE): A Randomised, Open‐Label, Phase 2 Trial,” Lancet Oncology 22, no. 12 (2021): 1705–1720, 10.1016/s1470-2045(21)00535-0. - DOI - PubMed
Publication types
MeSH terms
Grants and funding
- KJ2024CX045/Plan Project of Tongzhou Municipal Science and Technology
- 82170208/the National Natural Science Foundation of China
- 71003Y3035/Peking University Medicine Fund for world's leading discipline or discipline cluster development
- 2022YFC2502606/National Key Research and Development Program of China
- 82293630/Major Program of the National Natural Science Foundation of China
LinkOut - more resources
Full Text Sources
Medical
Research Materials

