Cyclosporine A Decreased Paclitaxel Resistance in Prostate Cancer Cells by Inhibiting MTDH Expression
- PMID: 39744936
- PMCID: PMC11694273
- DOI: 10.1177/15579883241310834
Cyclosporine A Decreased Paclitaxel Resistance in Prostate Cancer Cells by Inhibiting MTDH Expression
Abstract
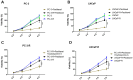
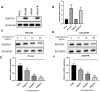
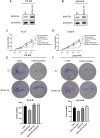
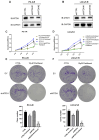
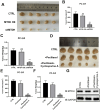
This study aims to investigate the effect and mechanism of cyclosporine A (CsA) on paclitaxel-resistant prostate cancer cells. Paclitaxel-resistant prostate cancer cell lines were established by gradual increment method. The proliferation of cells was tested using MTT and colony formation assay. Western blot was used to detect protein expression. Expression levels of gene mRNA were detected using real-time polymerase chain reaction (RT-PCR). Xenografts in nude mice were used to validate the conclusion in vitro. The results showed that CsA could increase the sensitivity of prostate cancer cells to paclitaxel. Treatment of paclitaxel-resistant prostate cancer cell lines with CsA gradients decreased metadherin (MTDH) protein expression. RT-PCR showed that CsA could decrease the mRNA level of MTDH. Overexpression of MTDH in prostate cancer cells increases paclitaxel resistance in prostate cancer cells. Conversely, knockdown of MTDH reduced paclitaxel resistance in prostate cancer cells. Treating cells with CsA failed to reduce paclitaxel resistance in prostate cancer cells when MTDH was overexpressed. Xenografts in nude mice yielded consistent conclusions with the in vitro results. In conclusion, CsA can reduce the resistance of prostate cancer cells to paclitaxel. In vitro and in vivo experiments have shown that CsA can reduce paclitaxel resistance in prostate cancer cells by decreasing MTDH expression. In clinical practice, CsA can be used in combination with paclitaxel to improve the therapeutic effect on prostate cancer. MTDH may serve as a novel target for treating paclitaxel resistance in prostate cancer.
Keywords: MTDH; cyclosporine A; drug resistance; paclitaxel; prostate cancer.
Conflict of interest statement
Declaration of Conflicting InterestsThe author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Figures
References
-
- Daniel P., Balušíková K., Truksa J., Černý J., Jaček M., Jelínek M., Mulenga M. J. V., Voráčová K., Chen L., Wei L., Sun Y., Ojima I., Kovář J. (2024). Effect of substituents at the C3′, C3′N, C10 and C2-meta-benzoate positions of taxane derivatives on their activity against resistant cancer cells. Toxicology and Applied Pharmacology, 489, Article 116993. 10.1016/j.taap.2024.116993 - DOI - PMC - PubMed
-
- Demaziere A., Mourgues C., Lambert C., Trevis S., Bertucat H., Grange I., Grange I., Pezet D., Sautou V., Jary M., Gagnière J. (2024). French multi-institutional cost-effectiveness analysis of gemcitabine plus nab-paclitaxel versus gemcitabine alone as second-line treatment in metastatic pancreatic cancer patients. Therapeutic Advances in Medical Oncology, 16, Article 259635. 10.1177/17588359241259635 - DOI - PMC - PubMed
-
- Ganugula R., Babalola K. T., Heyns I. M., Arora M., Agarwal S. K., Mohan C., Kumar M. N. V. R. (2024). Lymph node targeting of CsA ameliorates ocular manifestations in a mouse model of systemic lupus erythematosus (SLE) via PD-L1. Nano Today, 57, Article 102359. 10.1016/j.nantod.2024.102359 - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

