Cardiac fibroblast BAG3 regulates TGFBR2 signaling and fibrosis in dilated cardiomyopathy
- PMID: 39744939
- PMCID: PMC11684812
- DOI: 10.1172/JCI181630
Cardiac fibroblast BAG3 regulates TGFBR2 signaling and fibrosis in dilated cardiomyopathy
Abstract
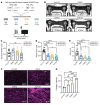
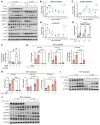
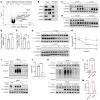
Loss of Bcl2-associated athanogene 3 (BAG3) is associated with dilated cardiomyopathy (DCM). BAG3 regulates sarcomere protein turnover in cardiomyocytes; however, the function of BAG3 in other cardiac cell types is understudied. In this study, we used an isogenic pair of BAG3-knockout and wild-type human induced pluripotent stem cells (hiPSCs) to interrogate the role of BAG3 in hiPSC-derived cardiac fibroblasts (CFs). Analysis of cell type-specific conditional knockout engineered heart tissues revealed an essential contribution of CF BAG3 to contractility and cardiac fibrosis, recapitulating the phenotype of DCM. In BAG3-/- CFs, we observed an increased sensitivity to TGF-β signaling and activation of a fibrogenic response when cultured at physiological stiffness (8 kPa). Mechanistically, we showed that loss of BAG3 increased transforming growth factor-β receptor 2 (TGFBR2) levels by directly binding TGFBR2 and mediating its ubiquitination and proteasomal degradation. To further validate these results, we performed single-nucleus RNA sequencing of cardiac tissue from DCM patients carrying pathogenic BAG3 variants. BAG3 pathogenic variants increased fibrotic gene expression in CFs. Together, these results extend our understanding of the roles of BAG3 in heart disease beyond the cardiomyocyte-centric view and highlight the ability of tissue-engineered hiPSC models to elucidate cell type-specific aspects of cardiac disease.
Keywords: Cardiology; Cardiovascular disease; Fibrosis; Human stem cells.
Figures


References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

