APP lysine 612 lactylation ameliorates amyloid pathology and memory decline in Alzheimer's disease
- PMID: 39744941
- PMCID: PMC11684803
- DOI: 10.1172/JCI184656
APP lysine 612 lactylation ameliorates amyloid pathology and memory decline in Alzheimer's disease
Abstract
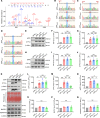
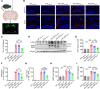
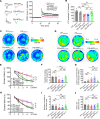
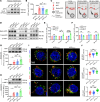
Posttranslational modification (PTM) of the amyloid precursor protein (APP) plays a critical role in Alzheimer's disease (AD). Recent evidence reveals that lactylation modification, as a novel PTM, is implicated in the occurrence and development of AD. However, whether and how APP lactylation contributes to both the pathogenesis and cognitive function in AD remains unknown. Here, we observed a reduction in APP lactylation in AD patients and AD model mice and cells. Proteomic mass spectrometry analysis further identified lysine 612 (APP-K612la) as a crucial site for APP lactylation, influencing APP amyloidogenic processing. A lactyl-mimicking mutant (APPK612T) reduced amyloid-β peptide (Aβ) generation and slowed down cognitive deficits in vivo. Mechanistically, APPK612T appeared to facilitate APP trafficking and metabolism. However, lactylated APP entering the endosome inhibited its binding to BACE1, suppressing subsequent cleavage. Instead, it promoted protein interaction between APP and CD2-associated protein (CD2AP), thereby accelerating the endosomal-lysosomal degradation pathway of APP. In the APP23/PS45 double-transgenic mouse model of AD, APP-Kla was susceptible to L-lactate regulation, which reduced Aβ pathology and repaired spatial learning and memory deficits. Thus, these findings suggest that targeting APP lactylation may be a promising therapeutic strategy for AD in humans.
Keywords: Aging; Alzheimer disease; Neurodegeneration; Neuroscience.
Conflict of interest statement
Figures




References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

