T helper 2 cell-directed immunotherapy eliminates precancerous skin lesions
- PMID: 39744942
- PMCID: PMC11684800
- DOI: 10.1172/JCI183274
T helper 2 cell-directed immunotherapy eliminates precancerous skin lesions
Abstract
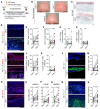
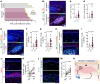
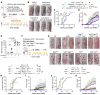
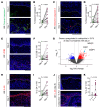
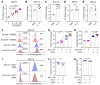
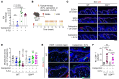
The continuous rise in skin cancer incidence highlights an imperative for improved skin cancer prevention. Topical calcipotriol-plus-5-fluorouracil (calcipotriol-plus-5-FU) immunotherapy effectively eliminates precancerous skin lesions and prevents squamous cell carcinoma (SCC) in patients. However, its mechanism of action remains unclear. Herein, we demonstrate that calcipotriol-plus-5-FU immunotherapy induces T helper type 2 (Th2) immunity, eliminating premalignant keratinocytes in humans. CD4+ Th2 cells were required and were sufficient downstream of thymic stromal lymphopoietin cytokine induction by calcipotriol to suppress skin cancer development. Th2-associated cytokines induced IL-24 expression in cancer cells, resulting in toxic autophagy and anoikis followed by apoptosis. Calcipotriol-plus-5-FU immunotherapy was dependent on IL-24 to suppress skin carcinogenesis in vivo. Collectively, our findings establish a critical role for Th2 immunity in cancer immunoprevention and highlight the Th2/IL-24 axis as an innovative target for skin cancer prevention and therapy.
Keywords: Dermatology; Immunotherapy; Oncology; Skin cancer; T cells.
Conflict of interest statement
Figures
Comment in
- Type 2 immunity to the rescue: enhancing antitumor immunity for skin cancer prevention doi: 10.1172/JCI188018
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

