Mechanisms of postischemic cardiac death and protection following myocardial injury
- PMID: 39744953
- PMCID: PMC11684816
- DOI: 10.1172/JCI184134
Mechanisms of postischemic cardiac death and protection following myocardial injury
Abstract
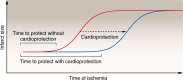
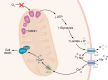
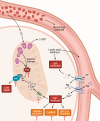
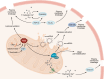
Acute myocardial infarction (MI) is a leading cause of death worldwide. Although with current treatment, acute mortality from MI is low, the damage and remodeling associated with MI are responsible for subsequent heart failure. Reducing cell death associated with acute MI would decrease the mortality associated with heart failure. Despite considerable study, the precise mechanism by which ischemia and reperfusion (I/R) trigger cell death is still not fully understood. In this Review, we summarize the changes that occur during I/R injury, with emphasis on those that might initiate cell death, such as calcium overload and oxidative stress. We review cell-death pathways and pathway crosstalk and discuss cardioprotective approaches in order to provide insight into mechanisms that could be targeted with therapeutic interventions. Finally, we review cardioprotective clinical trials, with a focus on possible reasons why they were not successful. Cardioprotection has largely focused on inhibiting a single cell-death pathway or one death-trigger mechanism (calcium or ROS). In treatment of other diseases, such as cancer, the benefit of targeting multiple pathways with a "drug cocktail" approach has been demonstrated. Given the crosstalk between cell-death pathways, targeting multiple cardiac death mechanisms should be considered.
Conflict of interest statement
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

