Engineering CAR-T Therapeutics for Enhanced Solid Tumor Targeting
- PMID: 39745117
- PMCID: PMC12160681
- DOI: 10.1002/adma.202414882
Engineering CAR-T Therapeutics for Enhanced Solid Tumor Targeting
Abstract
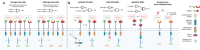
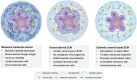
Cancer immunotherapy, specifically Chimeric Antigen Receptor (CAR)-T cell therapy, represents a significant breakthrough in treating cancers. Despite its success in hematological cancers, CAR-T exhibits limited efficacy in solid tumors, which account for more than 90% of all cancers. Solid tumors commonly present unique challenges, including antigen heterogeneity and complex tumor microenvironment (TME). To address these, efforts are being made through improvements in CAR design and the development of advanced validation platforms. While efficacy is limited, some solid tumor types, such as neuroblastoma and gastrointestinal cancers, have shown responsiveness to CAR-T therapy in recent clinical trials. In this review, it is first examined both experimental and computational strategies, such as protein engineering coupled with machine learning, developed to enhance T cell specificity. The challenges and methods associated with T cell delivery and in vivo reprogramming in solid tumors is discussed. It is also explored the advancements in engineered organoid systems, which are emerging as high-fidelity in vitro models that closely mimic the complex human TME and serve as a validation platform for CAR discovery. Collectively, these innovative engineering strategies offer the potential to revolutionize the next generation of CAR-T therapy, ultimately paving the way for more effective treatments in solid tumors.
Keywords: Chimeric antigen receptor (CAR); cancer immunotherapy; drug delivery; nanoparticle.
© 2025 The Author(s). Advanced Materials published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

