Targeting Cancer-Associated Fibroblasts: Eliminate or Reprogram?
- PMID: 39745128
- PMCID: PMC11875776
- DOI: 10.1111/cas.16443
Targeting Cancer-Associated Fibroblasts: Eliminate or Reprogram?
Abstract
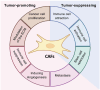
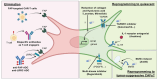
Cancer-associated fibroblasts (CAFs) are key components of the tumor microenvironment (TME). Given their various roles in tumor progression and treatment resistance, CAFs are promising therapeutic targets in cancer. The elimination of tumor-promoting CAFs has been investigated in various animal models to determine whether it effectively suppresses tumor growth. Based on recent evidence, several simple strategies have been proposed to eliminate tumor-promoting CAFs and attenuate these features. In addition, attention has focused on the critical role that CAFs play in the immunosuppressive TME. Therefore, the functional reprogramming of CAFs in combination with immune checkpoint inhibitors has also been investigated as a possible therapeutic approach. However, although potential targets in CAFs have been widely characterized, the plasticity and heterogeneity of CAFs complicate the understanding of their properties and present difficulties for clinical application. Moreover, the identification of tumor-suppressive CAFs highlights the necessity for the development of therapeutic approaches that can distinguish and switch between tumor-promoting and tumor-suppressive CAFs in an appropriate manner. In this review, we introduce the origins and diversity of CAFs, their role in cancer, and current therapeutic strategies aimed at targeting CAFs, including ongoing clinical evaluations.
Keywords: FAP; cancer‐associated fibroblasts; immunotherapy; reprogramming; tumor microenvironment.
© 2025 The Author(s). Cancer Science published by John Wiley & Sons Australia, Ltd on behalf of Japanese Cancer Association.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
Similar articles
-
IR-780 Dye-based Targeting of Cancer-associated Fibroblasts Improves Cancer Immunotherapy by Increasing Intra-tumoral T Lymphocytes Infiltration.Curr Cancer Drug Targets. 2024;24(6):642-653. doi: 10.2174/0115680096261142231018104854. Curr Cancer Drug Targets. 2024. PMID: 38310462
-
Immune checkpoint inhibitors as mediators for immunosuppression by cancer-associated fibroblasts: A comprehensive review.Front Immunol. 2022 Oct 5;13:996145. doi: 10.3389/fimmu.2022.996145. eCollection 2022. Front Immunol. 2022. PMID: 36275750 Free PMC article. Review.
-
Cancer-associated fibroblasts in clear cell renal cell carcinoma: functional heterogeneity, tumor microenvironment crosstalk, and therapeutic opportunities.Front Immunol. 2025 Jun 4;16:1617968. doi: 10.3389/fimmu.2025.1617968. eCollection 2025. Front Immunol. 2025. PMID: 40534854 Free PMC article. Review.
-
Drug Delivery System Targeting Cancer-Associated Fibroblast for Improving Immunotherapy.Int J Nanomedicine. 2025 Jan 11;20:483-503. doi: 10.2147/IJN.S500591. eCollection 2025. Int J Nanomedicine. 2025. PMID: 39816375 Free PMC article. Review.
-
Architecture of Cancer-Associated Fibroblasts in Tumor Microenvironment: Mapping Their Origins, Heterogeneity, and Role in Cancer Therapy Resistance.OMICS. 2020 Jun;24(6):314-339. doi: 10.1089/omi.2020.0023. OMICS. 2020. PMID: 32496970
Cited by
-
Revisiting the role of cancer-associated fibroblasts in tumor microenvironment.Front Immunol. 2025 Apr 17;16:1582532. doi: 10.3389/fimmu.2025.1582532. eCollection 2025. Front Immunol. 2025. PMID: 40313969 Free PMC article. Review.
-
In-depth insight into tumor-infiltrating stromal cells linked to tertiary lymphoid structures and their prospective function in cancer immunotherapy.Exp Hematol Oncol. 2025 Aug 10;14(1):105. doi: 10.1186/s40164-025-00695-8. Exp Hematol Oncol. 2025. PMID: 40784879 Free PMC article. Review.
-
Unveiling purine metabolism dysregulation orchestrated immunosuppression in advanced pancreatic cancer and concentrating on the central role of NT5E.Front Immunol. 2025 Apr 1;16:1569088. doi: 10.3389/fimmu.2025.1569088. eCollection 2025. Front Immunol. 2025. PMID: 40236698 Free PMC article.
References
-
- de Visser K. E. and Joyce J. A., “The Evolving Tumor Microenvironment: From Cancer Initiation to Metastatic Outgrowth,” Cancer Cell 41 (2023): 374–403. - PubMed
-
- Bu L., Baba H., Yoshida N., et al., “Biological Heterogeneity and Versatility of Cancer‐Associated Fibroblasts in the Tumor Microenvironment,” Oncogene 38 (2019): 4887–4901. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

