Metabolomics reveals alterations in gut-derived uremic toxins and tryptophan metabolism in feline chronic kidney disease
- PMID: 39745207
- PMCID: PMC11703532
- DOI: 10.1080/01652176.2024.2447601
Metabolomics reveals alterations in gut-derived uremic toxins and tryptophan metabolism in feline chronic kidney disease
Abstract
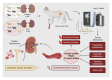
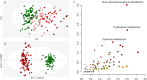
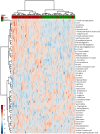
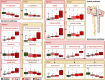
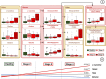
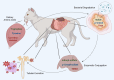
Chronic Kidney Disease (CKD) is one of the most common conditions affecting felines, yet the metabolic alterations underlying its pathophysiology remain poorly understood, hindering progress in identifying biomarkers and therapeutic targets. This study aimed to provide a comprehensive view of metabolic changes in feline CKD across conserved biochemical pathways and evaluate their progression throughout the disease continuum. Using a multi-biomatrix high-throughput metabolomics approach, serum and urine samples from CKD-affected cats (n = 94) and healthy controls (n = 84) were analyzed with ultra-high-performance liquid chromatography-high-resolution mass spectrometry. Significant disruptions were detected in tryptophan (indole, kynurenine, serotonin), tyrosine, and carnitine metabolism, as well as in the urea cycle. Circulating gut-derived uremic toxins, including indoxyl-sulfate, p-cresyl-sulfate, and trimethylamine-N-oxide, were markedly increased, primarily due to impaired renal excretion. However, alternative mechanisms, such as enhanced bacterial formation from dietary precursors like tryptophan, tyrosine, carnitine, and betaine, could not be ruled out. Overall, the findings suggest that metabolic disturbances in feline CKD are largely driven by the accumulation of gut-derived uremic toxins derived from precursors highly abundant in the feline diet. These insights may link the strict carnivorous nature of felines to CKD pathophysiology and highlight potential avenues for studying preventive or therapeutic interventions.
Keywords: Feline chronic kidney disease; biochemical pathway analysis; fundamental nephrology; gut microbiota derived uremic toxins; metabolomics; pathophysiology.
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures

References
-
- Bahn A, Ljubojevic M, Lorenz H, Schultz C, Ghebremedhin E, Ugele B, Sabolic I, Burckhardt G, Hagos Y.. 2005. Murine renal organic anion transporters mOAT1 and mOAT3 facilitate the transport of neuroactive tryptophan metabolites. Am J Physiol Cell Physiol. 289(5):C1075–1084. doi: 10.1152/ajpcell.00619.2004. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
