TOP1 Mutations and Cross-Resistance to Antibody-Drug Conjugates in Patients with Metastatic Breast Cancer
- PMID: 39745368
- PMCID: PMC12079096
- DOI: 10.1158/1078-0432.CCR-24-2771
TOP1 Mutations and Cross-Resistance to Antibody-Drug Conjugates in Patients with Metastatic Breast Cancer
Abstract
Purpose: Antibody-drug conjugates (ADC) harboring topoisomerase I (TOP1) inhibitor payloads have improved survival for patients with metastatic breast cancer. However, knowledge of ADC resistance mechanisms and potential impact on the sequential use of ADCs is limited. In this study, we report the incidence and characterization of TOP1 mutations arising in the setting of ADC resistance in metastatic breast cancer.
Experimental design: Patients with metastatic breast cancer treated with ADCs with available posttreatment plasma-based genotyping were included. TOP1 mutation incidence, mutant allele frequency, and functional characterization were assessed, and incidence was compared with that in patients with metastatic breast cancer not receiving ADC treatment and in The Cancer Genome Atlas.
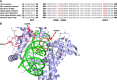
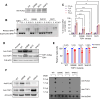
Results: Plasma-based genotyping identified distinct TOP1 mutations (S57C, R364H, W401C, and G359E) in 12.9% of patients (4/31) at the time of disease progression on ADC, compared with 0.7% (3/420) in non-ADC-treated patients with metastatic breast cancer and 0.5% in The Cancer Genome Atlas. The appearance of mutations was associated with clinical cross-resistance, as median duration on the first ADC was 455 versus 52 days for the second ADC. The functional characterization of three novel TOP1-mutant proteins demonstrated that all exhibited reduced enzymatic activity, attenuated covalent DNA binding, and resistance to TOP1 inhibitor ADC payloads SN38 and deruxtecan.
Conclusions: We describe the recurrent emergence of functionally altered, resistance-associated TOP1 mutations in vivo under selective pressure from ADCs and the potential impact on mediating cross-resistance to sequential ADCs. TOP1 mutation may represent a biomarker of resistance in this setting, and additional work is needed to optimize biomarkers and ADC payload design to improve outcomes for the sequential use of ADCs. See related commentary by Gwin and Hurvitz, p. 1824.
©2025 The Authors; Published by the American Association for Cancer Research.
Conflict of interest statement
R.O. Abelman reports a grant from the Conquer Cancer Foundation during the conduct of the study. B. Wu reports a grant from the Terri Brodeur Breast Cancer Foundation during the conduct of the study. A. Medford reports personal fees from AstraZeneca, Edgewood Oncology, Guardant Health, Illumina, Myriad Genetics, Natera, and Science for America and a grant from the National Institutes of Health (K12) outside the submitted work. C. Weipert is an employee and a stockholder of Guardant Health, Inc. L.M. Spring reports service as a consultant/service on the advisory board of Novartis, Daiichi Pharma, AstraZeneca, Eli Lilly, Precede, and Seagen; institutional research support from Merck, Genentech, Gilead, Eli Lilly, and AstraZeneca; and travel support from Eli Lilly. S.A. Wander reports personal fees from Foundation Medicine, Veracyte, Hologic, Biovica, Novartis, and AstraZeneca; personal fees and other support from Eli Lilly, Pfizer/Arvinas, Puma Biotechnology, Genentech, Regor Therapeutics, and Stemline/Menarini; and other support from Sermonix, Guardant Health, and 2ndMD outside the submitted work. N. Vidula reports grants from Merck, Radius, Daehwa, Novartis, Pfizer, and Stemline and personal fees from Gilead, Aadi, TerSera, Novartis, and IDEOlogy Health outside the submitted work. D. Juric reports grants and personal fees from Novartis, Genentech, Syros, Eisai, Pfizer, and Takeda; personal fees from Vibliome, PIC Therapeutics, Mapkure, and Relay Therapeutics; and grants from Amgen, InventisBio, Arvinas, Blueprint, AstraZeneca, Ribon Therapeutics, and Scorpion Therapeutics outside the submitted work. R.B. Corcoran reports personal fees and other support from Sidewinder Therapeutics, Alterome Therapeutics, Pheon Therapeutics, C4 Therapeutics, Cogent Biosciences, Erasca, Kinnate Biopharma, Nested Therapeutics, nRichDx, Remix Therapeutics, and Revolution Medicines; personal fees from AbbVie, Amgen, Bristol Myers Squibb, Elicio, Parabilis Medicines, Genentech/Roche, Mirati Therapeutics, Qiagen, and Taiho; and grants from Invitae, Novartis, Relay Therapeutics, and OnKure outside the submitted work. L.W. Ellisen reports a grant from National Institutes of Health (R01) and the Congressionally Directed Medical Research Programs (CDMRP) during the conduct of the study as well as personal fees from Gilead, AstraZeneca, Atavistik, and Kisoji and other support from Sanofi outside the submitted work; in addition, L.W. Ellisen reports a patent for PCT/US2022/079958 pending. A. Bardia reports a grant from National Institutes of Health (R01) and the CDMRP and grants and personal fees from Pfizer, Novartis, Genentech, Merck, Menarini, Gilead, Sanofi, AstraZeneca, Daiichi Sankyo, and Eli Lilly during the conduct of the study, as well as a patent for Trop 2 ADC and PARPi pending. No disclosures were reported by the other authors.
Figures

References
-
- Shastry M, Gupta A, Chandarlapaty S, Young M, Powles T, Hamilton E. Rise of antibody-drug conjugates: the present and future. Am Soc Clin Oncol Educ Book 2023;43:e390094. - PubMed
-
- Bardia A, Hurvitz SA, Tolaney SM, Loirat D, Punie K, Oliveira M, et al. Sacituzumab govitecan in metastatic triple-negative breast cancer. N Engl J Med 2021;384:1529–41. - PubMed
-
- Abelman RO, Spring L, Fell GG, Ryan P, Vidula N, Medford AJ, et al. Sequential use of antibody-drug conjugate after antibody-drug conjugate for patients with metastatic breast cancer: ADC after ADC (A3) study. J Clin Oncol 2023;41(Suppl 16):1022.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

