Biophysical and Structural Features of αβT-Cell Receptor Mechanosensing: A Paradigmatic Shift in Understanding T-Cell Activation
- PMID: 39745432
- PMCID: PMC11744257
- DOI: 10.1111/imr.13432
Biophysical and Structural Features of αβT-Cell Receptor Mechanosensing: A Paradigmatic Shift in Understanding T-Cell Activation
Abstract
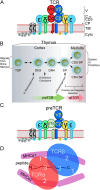
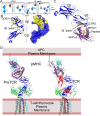
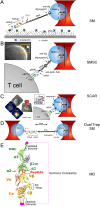
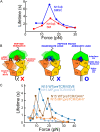
αβT cells protect vertebrates against many diseases, optimizing surveillance using mechanical force to distinguish between pathophysiologic cellular alterations and normal self-constituents. The multi-subunit αβT-cell receptor (TCR) operates outside of thermal equilibrium, harvesting energy via physical forces generated by T-cell motility and actin-myosin machinery. When a peptide-bound major histocompatibility complex molecule (pMHC) on an antigen presenting cell is ligated, the αβTCR on the T cell leverages force to form a catch bond, prolonging bond lifetime, and enhancing antigen discrimination. Under load, the αβTCR undergoes reversible structural transitions involving partial unfolding of its clonotypic immunoglobulin-like (Ig) domains and coupled rearrangements of associated CD3 subunits and structural elements. We postulate that transitions provide critical energy to initiate the signaling cascade via induction of αβTCR quaternary structural rearrangements, associated membrane perturbations, exposure of CD3 ITAMs to phosphorylation by non-receptor tyrosine kinases, and phase separation of signaling molecules. Understanding force-mediated signaling by the αβTCR clarifies long-standing questions regarding αβTCR antigen recognition, specificity and affinity, providing a basis for continued investigation. Future directions include examining atomistic mechanisms of αβTCR signal initiation, performance quality, tissue compliance adaptability, and T-cell memory fate. The mechanotransduction paradigm will foster improved rational design of T-cell based vaccines, CAR-Ts, and adoptive therapies.
Keywords: T cell; T‐cell receptor (TCR); cell signaling; mechanosensing; molecular dynamics (MD); optical tweezers (OT); preTCR; single molecule (SM).
© 2024 The Author(s). Immunological Reviews published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures




References
-
- Reinherz E. L., Hussey R. E., and Schlossman S. F., “A Monoclonal Antibody Blocking Human T Cell Function,” European Journal of Immunology 10 (1980): 758–762. - PubMed
-
- Allison J. P., McIntyre B. W., and Bloch D., “Tumor‐Specific Antigen of Murine T‐Lymphoma Defined With Monoclonal Antibody,” Journal of Immunology 129 (1982): 2293–2300. - PubMed
-
- Meuer S. C., Acuto O., Hussey R. E., et al., “Evidence for the T3‐Associated 90K Heterodimer as the T‐Cell Antigen Receptor,” Nature 303 (1983): 808–810. - PubMed
-
- Hedrick S. M., Cohen D. I., Nielsen E. A., and Davis M. M., “Isolation of cDNA Clones Encoding T Cell‐Specific Membrane‐Associated Proteins,” Nature 308 (1984): 149–153. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

