Octahedral small virus-like particles of dengue virus type 2
- PMID: 39745459
- PMCID: PMC11853069
- DOI: 10.1128/jvi.01809-24
Octahedral small virus-like particles of dengue virus type 2
Abstract
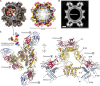
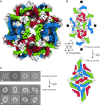
Flavivirus envelope (E) and precursor M (prM) proteins, when ectopically expressed, assemble into empty, virus-like particles (VLPs). Cleavage of prM to M and loss of the pr fragment converts the VLPs from immature to mature particles, mimicking a similar maturation of authentic virions. Most of the VLPs obtained by prM-E expression are smaller than virions; early, low-resolution cryo-EM studies suggested a simple, 60-subunit, icosahedral organization. We describe here the cryo-EM structure of immature, small VLPs (smVLPs) from dengue virus type 2 and show that they have octahedral rather than icosahedral symmetry. The asymmetric unit of the octahedral particle is an asymmetric trimer of prM-E heterodimers, just as it is on icosahedral immature virions; the full, octahedrally symmetric particle thus has 24 such asymmetric trimers or 72 prM-E heterodimers in all. Cleavage of prM and release of pr generates ovoid, somewhat irregular, mature particles. Previous work has shown that mature smVLPs have fusion properties identical to those of virions, consistent with local, virion-like clustering of 36 E dimers on their surface. The cryo-EM structure and the properties of the smVLPs described here relate directly to ongoing efforts to use them as vaccine immunogens.
Importance: Ectopic expression of flavivirus envelope (E) and precursor M (prM) proteins leads to the formation and secretion of empty, virus-like particles (VLPs). We show that a major class of VLPs, of smaller diameter than those of virion size ("small VLPs": smVLPs), are octahedrally symmetric particles. The known characteristics of immature virions (asymmetric trimers of prM-E heterodimers) allow us to understand the assembly of an octahedral (rather than icosahedral) surface lattice. Cleavage of prM and formation of mature, fusogenic smVLPs yield somewhat irregular, ovoid particles. These observations are directly relevant to proposals for using immunogenic but non-infectious VLPs as components of specific flavivirus vaccines.
Keywords: cryo-EM; flavivirus; structure; vaccine.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Newton ND, Hardy JM, Modhiran N, Hugo LE, Amarilla AA, Bibby S, Venugopal H, Harrison JJ, Traves RJ, Hall RA, Hobson-Peters J, Coulibaly F, Watterson D. 2021. The structure of an infectious immature flavivirus redefines viral architecture and maturation. Sci Adv 7. doi: 10.1126/sciadv.abe4507 - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

